| Research Article | ||
J. Microbiol. Infect. Dis., (2024), Vol. 14(2): 55–59 Research Article Ralstonia mannitolilytica septicaemia, an added burden in cancer care-recognizing the unrecognizedSreedhar Jayakrishnan Cherulil1*, K. V. Gangadharan1, Arun Chandrashekaran1, K. P. Sreelesh1 and M. R. Kesavan21Department of Medical Oncology, ASTER MIMS, Kozhikode, India 2Department of Paediatric Haematology, ASTER MIMS, Kozhikode, India *Corresponding Author: Sreedhar Jayakrishnan Cherulil. Department of Medical Oncology, ASTER MIMS, Kozhikode, India. Email: srj1216353 [at] gmail.com Submitted: 01/03/2024 Accepted: 04/06/2024 Published: 30/06/2024 © 2024 Journal of Microbiology and Infectious Diseases
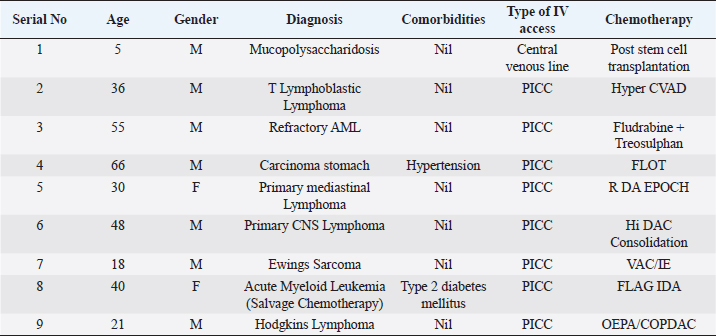
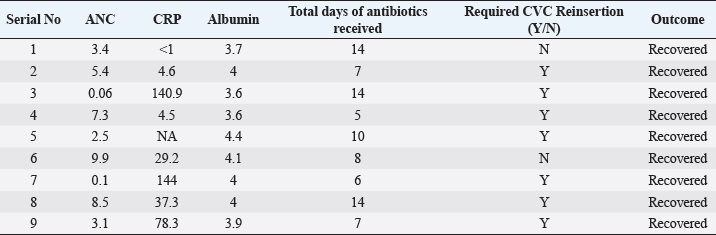
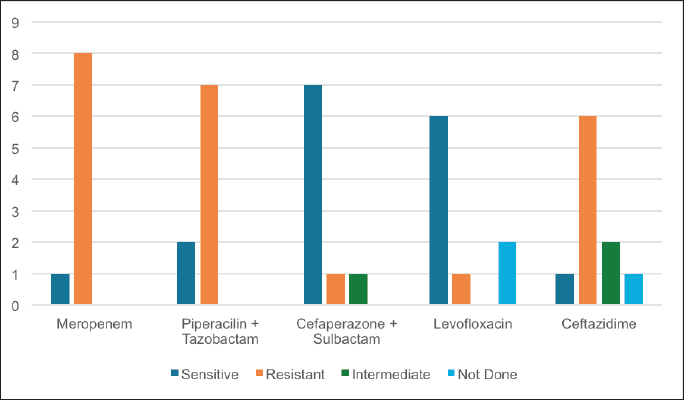
ABSTRACTBackground: Ralstonia mannitolilytica is an often underrecognized pathogen, that can cause infections in immunocompromised hosts. Indwelling catheters are being increasingly used in cancer patients, R. mannitolilytica has a propensity to form biofilms, and this makes it an important pathogen in catheter-related infections. In this study, we report on an outbreak of Ralstonia septicaemia at our facility. Aim: To characterize an outbreak of Ralstonia infections at a tertiary care facility with a focus on antibiotic sensitivity and added burden on treatment. Design: A retrospective analysis of an outbreak of sepsis attributed to R. mannitolilytica. Methods: We report on nine cases of proven R. mannitolilytica septicaemia in the period from January 2022 to June 2023. Results: Blood cultures were positive in 66.7%, and three had tip cultures positive. 77.8% of the patients required removal of their central venous access, due to hemodynamic compromise and/or persistent fever despite adequate coverage with empirical antibiotics. 85.7% required reinsertion of PICC lines. Eight of the isolates (88.9%) were resistant to Meropenem, and seven were resistant to Piperacilin + Tazobactam (77.8%). Resistance to Ceftazidime was seen in six isolates, Sensitivity to Cefaperazone + Sulbactam, and Levolfloxacin was seen in seven and six patients, respectively. IntroductionInfections remain one of the most challenging aspects of cancer care, and the emergence of antibiotic resistance further complicates the management of septicaemia. Antibiotic failure contributes to an increase of sepsis-related mortality and significantly adds to the cost of care (Nanayakkara et al., 2021). The issue of antibiotic resistance has always been a concern in the oncology community, with the presence of bacteraemia significantly impacting mortality. Mortality rates approaching 11% have been observed in patients with haematological malignancies with febrile neutropenia (John et al., 2023). The use of central venous access is commonplace for delivering therapy in both solid and haematological malignancies, and these can act as the nidus for catheter-associated blood stream infections (CLABSI). CLABSI also adds to the financial toxicity of cancer treatment. There are regional and local variations in the spectrum of organisms responsible for bacteraemia in cancer patients and those with CLABSI. Indian studies have shown Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa to be the most common organisms isolated in cases of neutropenic sepsis (John et al, 2023; Ramani et al, 2021; Mishra et al, 2017). Non-fermenting gram-negative rods have been commonly associated with nosocomial infections. There are several opportunistic pathogens in this group, including Acinetobacter, Stenotrophomonas maltophilia, and so on. The genus Ralstonia, has emerged as an opportunistic pathogen of concern in recent years, with outbreaks of bloodstream infections being reported from day care oncology care facilities, along with reports of recurrent meningitis and other forms of infections (Owusu et al., 2019; Ryan, and Adley, 2014). The genus Ralstonia contains three clinically relevant species, Ralstonia pickettii, Ralstonia insidiosa and Ralstonia mannitolilytica, of these R. pickettii is the more commonly implicated species in several reports (Dotis et al., 2012; Liu et al., 2016). More recent evidence has pointed towards R. mannitolilytica also being an emerging cause of hospital acquired infections, with reports of outbreaks of bacteraemia associated with contamination of parenteral fluids and saline infusions prepared in hospital pharmacies (Lucarelli et al., 2017). The ability of these organisms to form biofilms and pass through 0.2 micron filters and their propensity to form biofilms, along with the documented survival of these organisms in saline and sanitizer solutions make them an organism of concern, especially in cases of outbreaks of CLABSI (Ryan, Adley et al., 2014; Lucarelli et al., 2017). Our study is a retrospective analysis of a cluster of R. mannitolilytica infections at a tertiary cancer care facility, aimed at characterizing the sensitivity patterns of the organism and the impact of the infection on the course of treatment. Materials and MethodsA total of nine documented cases of R. mannitolilytica infection was observed in the period from January 2022 to June 2023. All patients had presented with symptoms of fever and/or chills, with or without signs of shock. All patients had blood cultures drawn, both from the PICC line/ Chemoport and a peripheral set of blood cultures. Patients who had to have their indwelling catheters removed, a further tip culture of the removed catheter was also obtained. The demographic characteristics of the patients were documented, and once the cultures were obtained, the antibiotic sensitivity patterns were also noted. The duration of hospitalization and the possible delays in initiation of chemotherapy due to the infection were also looked at. An epidemiological survey to identify the source of infection was also carried out. Surveillance cultures were taken from the sanitizer solutions used for the patients, along with cultures from batches of IV fluids used for the patients, along with cultures taken from the sterile water solutions used for injection were also obtained. All surveillance cultures followed the same procedure as that used for the primary identification of the organism. Isolation and IdentificationBlood cultures were taken from both the peripheral vein and chemoport or CVC line. These were tested on the Bactalert 3D system. After growth was detected, they were plated on Mac Conkey & Sheep Blood (5%–7%) agar plates. Subsequently, the plates was incubated aerobically at 37°C +/−1°C for 24–48 hours. Colonies were then put on ViTek 2 Compact System (Biomerieux) using Vitek 2GN card (The VITEK 2 system has been validated for the identification of non-fermenting rods, correctly identifying above 90% of the strains). Manual sensitivity was put up for all the isolates. Mueller Hinton agar was used for testing of antibiotic discs the discs were put up as per Clinical and Laboratory Standards Institute (CLSI) guidelines. As there were no CLSI breakpoints or zones available for Ralstonia, results were interpreted using CLSI criteria for Pseudomonas and Burkholderia cepacia complex. ResultsWe had a total of nine patients who were culture positive for R. mannitolilytica, in this retrospective analysis. All the patients had presented with fever or developed fever during admission. All patients had central venous access, eight of the patients (88.9%) had a PICC (Peripherally Inserted Central Venous Catheter), and one patient had a jugular central venous line. Baseline characteristics of the patients are shown in Table 1. The median age of our population was 38 years (5–65 years). Seven patients were male and two were females. All patients had cultures positive for R. manitolilytica, 66.7% (6 out of the 9 patients) had blood cultures positive, while three patients had tip cultures of the PICC lines positive for the organism. Only two patients were neutropenic at the time of becoming culture positive for R. mannitolilytica. All patients recovered from the infectious episode, and none of the patients required admission to the intensive care unit during the period of hospitalization (Table 2). The median duration of antibiotic use was eight days, while three out of the nine patients (33.3%) had a greater than 1 week delay in administration of chemotherapy. 77.8% of the patients required removal of their central venous access, due to hemodynamic compromise and/or persistent fever despite adequate coverage with empirical antibiotics. Out of the seven patients who had their central venous access removed, six patients (85.7%) required reinsertion of a new PICC line for continuation of chemotherapy. Antibiotic sensitivity patterns analysis (Fig. 1) showed that eight of the isolates (88.9%) were resistant to Meropenem, and seven isolates were resistant to Piperacilin + Tazobactam (77.8%). Resistance to Ceftazidime was seen in six isolates, while seven were found to be sensitive to Cefaperazone + Sulbactam, and six isolates were sensitive to Levofloxacin. An epidemiological survey was conducted, but it failed to identify a source of infection. Table 1. Baseline characteristics of patients.
Table 2. Treatment details and outcomes of patients.
Fig. 1. Antibiotic sensitivity patterns to commonly used antibiotics. DiscussionRalstonia is an environmental pathogen, that is associated with hospital acquired infections. It has not been associated with life threatening infections but is being increasingly isolated as a cause of infections in immunocompromised patients (Gröbner et al., 2007). Several reports of outbreaks of infections attributed to Ralstonia species have been reported in literature. Reports of Ralstonia infections have also implicated the microbe in serious infections such as recurrent meningitis, myelitis and peritonitis (Fernandez et al., 1996; Ryan et al., 2014). Several properties of the microbe, makes it a particularly dangerous pathogen in patients with indwelling catheters. The microbe can pass through 0.2 micron filters used, as well as the ability to form biofilms (Daxboeck et al., 2005; Ryan et al., 2014). Gobner et al, described an outbreak, of catheter related infections due to R. mannitolilytica in haemato – oncology wards, and concluded that the source of infection was possibly contaminated IV solutions (Coman et al., 2017). R.picketti, another member of the genus, has been shown to be able to survive in 0.05% chlorhexidine solutions, and even contaminate sterile medical products like intravenous (IV) solutions, Ranitidine IV preparations, and respiratory solutions (Fernandez et al., 1996; Coman et al., 2017). A similar ability to survive in such environments has also been attributed to R. mannitolilytica. Lucarelli et al. (2017) reported a series of 22 cases of R. mannitolilytica in an oncology day care unit, they had implicated the use of multiuse vials as a possible source of the pathogen. Several reports of outbreaks of Ralstonia infections have reported prolonged duration of outbreaks, including Lucarelli et al. (2017), Gröbner et al. (2007) who reported an outbreak that lasted 11 weeks and Daxboeck et al, who reported on cases that spanned a duration of 2 years (Daxboeck et al., 2005; Gröbner et al., 2007; Coman et al., 2017). Another study by Ramani et al. (2021) had a gap of 60 days between the first and last isolate, we have also had a significant time interval between the first and last isolates in our study population (Ryan, Adley, 2014). Several previous accounts of Ralstonia outbreaks have undertaken epidemiological surveys and have been subsequently unable to isolate the pathogen from any of the environmental sources, the same has been the case in our analysis. In keeping with most case series and reports on Ralstonia infections, all our patients had complete recovery from their infectious episodes, Ramani et al. (2021) Lucarelli et al. (2017) have all reported complete recovery of all patients. A study by Coman et al.(2017) has shown Ralstonia infections to be a predictor of dramatic worsening of outcomes in patients of cystic fibrosis, but the same has not been noted in oncological cases (Ryan and Adley CC 2014; Gröbner et al., 2007; Coman et al., 2017). The antibiotic sensitivity patterns showed a high percentage of resistance to meropenem, which has again been noted in several case series. Carbanem resistance was reported by Daxboeck et al in 40% of their patients, while Lucarelli et al. (2017) could not identify any carbapenamese genes, but they suggested an alternative mechanism for the resistance to meropenem observed in their study mediated by other beta lactamases (Gröbner et al., 2007; Daxboeck et al., 2005). The high percentage of patients who required a re-insertion of their central venous access catheters, shows the significant financial toxicity that nosocomial infections with Ralstonia species entails. Ramani et al. (2021) estimated an additional burden of USD 650–800 in view of hospitalization and supportive care for patients, while the removal and reinsertion of CVC devices was estimated to further entail a cost of USD 330 per patient (Ryan and Adley, 2014). The institution of several changes in the infection control policy, which included changing the batches of IV solutions and disinfectant solutions as well the sterile water solutions used has probably contributed to there being no further detected cases of Ralstonia infections in our experience. Thus, in conclusion, while R. mannitolilytica is not a life-threatening infection, it represents a significant pathogen for immunocompromised patients and those undergoing chemotherapy, especially those with indwelling catheters. The financial toxicity entailed because of the infection makes prevention vitally important, and the introduction of appropriate infection control measures plays a significant role in tackling this emerging infection. AcknowledgmentThe would like to acknowledge the team of clinical pharmacists (Muhammed Fayiz and Maria Joseph) and the infection control staff. Authors contributionsAll authors contributed to the study. All authors read and accepted the final manuscript. FundingNone Conflict of interestThe authors declare that there is no conflict of interest. Data AvailabilityAll data is provided in the manuscript. ReferencesComan I, Bilodeau L, Lavoie A, Carricart M, Tremblay F, Zlosnik J E and Berthiaume Y 2017. Ralstonia mannitolilytica in cystic fibrosis: a new predictor of worse outcomes. Resp Med Case Rep. 20:48–50. Daxboeck F, Stadler M, Assadian O, Marko E, Hirschl A M and Koller W. 2005. Characterization of clinically isolated Ralstonia mannitolilytica strains using random amplification of polymorphic DNA (RAPD) typing and antimicrobial sensitivity, and comparison of the classification efficacy of phenotypic and genotypic assays. J Med Microbiol. 54(1):55–61. Demirdag T B, Ozkaya-Parlakay A, Bayrakdar F, Gulhan B, Yuksek S K and Yildiz S S, et al. 2022. An outbreak of Ralstonia pickettii bloodstream infection among pediatric leukemia patients. J Microbiol Immunol Infect. 55(1):80–5. Dotis J, Printza N, Orfanou A, Papathanasiou E and Papachristou F. 2012. Peritonitis due to Ralstonia mannitolilytica in a pediatric peritoneal dialysis patient. Microbiol-Q J Microbiol Sci. 35(4):503. Fernandez C, Wilhelmi I, Andradas E, Gaspar C, Gomez J, Romero J, Mariano J A, Corral O, Rubio M, Elviro J and Fereres J. 1996. Nosocomial outbreak of Burkholderia pickettii infection due to a manufactured intravenous product used in three hospitals. Clin Infect Dis. 22(6):1092–5. Gröbner S, Heeg P, Autenrieth I B and Schulte B. 2007. Monoclonal outbreak of catheter-related bacteraemia by Ralstonia mannitolilytica on two haemato-oncology wards. J Infect. 55(6):539–44. John K R, Warrier A and Warrier A. 2023. Microbiological spectrum of neutropenic Sepsis in cancer patients admitted to a tertiary Health Care Centre. Cureus. 158. Lucarelli C, Di Domenico E G, Toma L, Bracco D, Prignano G, Fortunati M, Pelagalli L, Ensoli F, Pezzotti P, García-Fernández A, Pantosti A and Ingrosso L. 2017. Ralstonia mannitolilytica infections in an oncologic day ward: description of a cluster among high-risk patients. Antimicrob Resist Infect Control. 6:1–7. Liu CX, Yan C, Zhang P, Li FQ, Yang JH and Li XY. 2016. Ralstonia mannitolilytica-induced septicemia and homology analysis in infected patients: 3 case reports. Jundishapur J Microbiol. 9(7). Mishra S B, Misra R, Azim A, Baronia A K, Prasad K N, Dhole T N, Gurjar M, Singh R K and Poddar B. 2017. Incidence, risk factors and associated mortality of central line-associated bloodstream infections at an intensive care unit in northern India. Int J Qual Health Care. 29(1):63–7. Marroni M, Pasticci M B, Pantosti A, Colozza M A, Stagni G and Tonato M. 2003. Outbreak of infusion-related septicemia by Ralstonia pickettii in the oncology department. Tumori J. 89(5):575–6. Nanayakkara A K, Boucher H W, Fowler Jr V G, Jezek A, Outterson K and Greenberg D E. 2021. Antibiotic resistance in the patient with cancer: escalating challenges and paths forward. CA Cancer J Clin. 71(6):488–504. Owusu M, Acheampong G, Annan A, Marfo K S, Osei I, Amuasi J, Sarpong N, Im J, Mogeni O D, Chiang H Y, Kuo C H, Jeon H J, Panzner U, Park S E, Marks F, Owusu-Dabo E and Adu-Sarkodie Y2019. Ralstonia mannitolilytica sepsis: a case report. J Med Case Rep. 13(1):1–4. Ramani V K, Ganesha D V, Sarathy V, Bhattacharjee S, Ganeshan S and Naik R. 2021. Outbreak of Ralstonia mannitolilytica infection at a tertiary care oncology center in South India: a case series. Asia Pac J Cancer Biol. 6(1):87–92. Ryan M P and Adley C C. 2014. Ralstonia spp.: emerging global opportunistic pathogens. European J Clin Microbiol Infect Dis. 33:291–304. | ||
| How to Cite this Article |
| Pubmed Style Cherulil SJ, Gangadharan KV, Chandrashekaran A, Sreelesh KP, Kesavan MR. Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized. J Microbiol Infect Dis. 2024; 14(2): 55-59. doi:10.5455/JMID.2024.v14.i2.3 Web Style Cherulil SJ, Gangadharan KV, Chandrashekaran A, Sreelesh KP, Kesavan MR. Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized. https://www.jmidonline.org/?mno=192745 [Access: January 25, 2026]. doi:10.5455/JMID.2024.v14.i2.3 AMA (American Medical Association) Style Cherulil SJ, Gangadharan KV, Chandrashekaran A, Sreelesh KP, Kesavan MR. Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized. J Microbiol Infect Dis. 2024; 14(2): 55-59. doi:10.5455/JMID.2024.v14.i2.3 Vancouver/ICMJE Style Cherulil SJ, Gangadharan KV, Chandrashekaran A, Sreelesh KP, Kesavan MR. Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized. J Microbiol Infect Dis. (2024), [cited January 25, 2026]; 14(2): 55-59. doi:10.5455/JMID.2024.v14.i2.3 Harvard Style Cherulil, S. J., Gangadharan, . K. V., Chandrashekaran, . A., Sreelesh, . K. P. & Kesavan, . M. R. (2024) Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized. J Microbiol Infect Dis, 14 (2), 55-59. doi:10.5455/JMID.2024.v14.i2.3 Turabian Style Cherulil, Sreedhar Jayakrishnan, K. V. Gangadharan, Arun Chandrashekaran, K. P. Sreelesh, and M. R. Kesavan. 2024. Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized. Journal of Microbiology and Infectious Diseases, 14 (2), 55-59. doi:10.5455/JMID.2024.v14.i2.3 Chicago Style Cherulil, Sreedhar Jayakrishnan, K. V. Gangadharan, Arun Chandrashekaran, K. P. Sreelesh, and M. R. Kesavan. "Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized." Journal of Microbiology and Infectious Diseases 14 (2024), 55-59. doi:10.5455/JMID.2024.v14.i2.3 MLA (The Modern Language Association) Style Cherulil, Sreedhar Jayakrishnan, K. V. Gangadharan, Arun Chandrashekaran, K. P. Sreelesh, and M. R. Kesavan. "Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized." Journal of Microbiology and Infectious Diseases 14.2 (2024), 55-59. Print. doi:10.5455/JMID.2024.v14.i2.3 APA (American Psychological Association) Style Cherulil, S. J., Gangadharan, . K. V., Chandrashekaran, . A., Sreelesh, . K. P. & Kesavan, . M. R. (2024) Ralstonia mannitolilytica septicaemia, an added burden in cancer care: Recognizing the unrecognized. Journal of Microbiology and Infectious Diseases, 14 (2), 55-59. doi:10.5455/JMID.2024.v14.i2.3 |