| Research Article | ||
J. Microbiol. Infect. Dis., (2024), Vol. 14(2): 38–45 Research Article Infants with 4-5 years after intrauterin exposition to zika virus have risk of neurodevelopmental alterationsArlene G. Acosta-Mass1, David E. Calvillo-Cervantes2, Gilma G. Sánchez-Burgos3*1Instituto Mexicano del Seguro Social, Hospital General de Zona con Unidad de Medicina Familiar “Dr. Abraham Azar Farah”, Campeche, México. 2Instituto Mexicano del Seguro Social, Hospital General Regional No 1 “Lic. Ignacio García Téllez”, Mérida, México 3Instituto Mexicano del Seguro Social, Unidad de Investigación Médica Yucatán, Mérida, México *Corresponding Author: Gilma G. Sánchez-Burgos. Instituto Mexicano del Seguro Social, Unidad de Investigación Médica Yucatán, Mérida, México. E-mail: gilmagburgos [at] gmail.com. Submitted: 13/02/2024 Accepted: 18/06/2024 Published: 30/06/2024 © 2024 Journal of Microbiology and Infectious Diseases
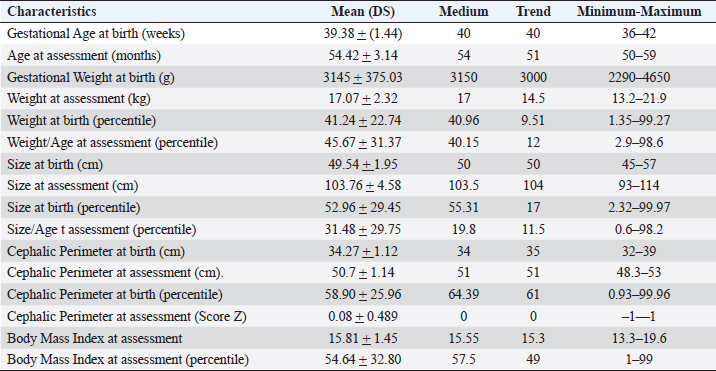
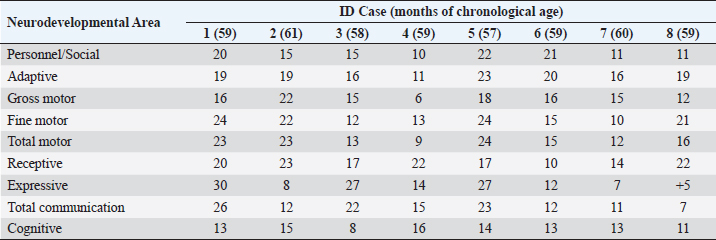
ABSTRACTBackground: During 2016-2020, 12,955 cases of Zika virus (ZIKV) infections were reported in México, where Yucatán was the second state with a high prevalence. However, the neurological development of children with gestational exposure to these viruses has been poorly studied. Aim: We aim to describe the neurodevelopmental status of infants exposed to ZIKV during pregnancy in Yucatán, México. Methods: Infants aged 4-5 years exposed in utero to ZIKV were first evaluated with the Child Development Assessment 2nd version test (EDI-ll) and later with the Battelle Inventory 2nd version test (IDB-ll). The anthropometric characteristics were evaluated using the Intergrowth 21st Calculator application, the Body Mass Index calculator developed by the CDC, and the WHO head circumference chart. Results: Fifty infants without microcephaly were included, the median age of 54.42 ± 3.14 months; 28% presented lag, and 14% showed a risk of delay. The Fine Motor and Communication areas were the most affected (24% and 20%, respectively), followed by Language (16%), Social (12%), and Gross Motor (8%). At reassessment, 64.3% were reclassified as normal and 14.3% remained at risk of delay. The IDB-II test identified neurodevelopmental lags of 10–20 months to chronological ages, with a greater lag in the Expressive area and a smaller lag in the Cognitive area. A higher frequency of neurodevelopmental disorders was found in the second trimester of pregnancy. However, exposure during the first trimester of pregnancy coincided with a higher frequency of risk of delay. Conclusion: Our results emphasize the need to evaluate the neurodevelopment of infants with antenatal exposition to ZIKV, during the first 5 years of life from birth to improve their future quality of life. Key Words: Infants, Gestation, Neurodevelopment, México, Zika virus IntroductionIn 2015, the zika virus (ZIKV) was associated with neurological alterations such as myelitis, meningoencephalitis, and encephalomyelitis in infected people (de Araújo et al., 2016; de Oliveira et al., 2017). In 2016, the first case of microcephaly associated with congenital ZIKV infection was confirmed in México, where caused 12,955 autochthonous cases until epidemiological week 11 of 2021, mostly in the state of Yucatán (Hernández-Ávila et al., 2018; Secretaría de Salud, 2021). Of the 1,325 confirmed cases in Yucatán, 69.88% (926 cases) occurred in pregnant women (Secretaría de Salud, 2021). Brain architecture develops during the first 3–5 years of life, favoring the development of the senses, learning, memory, and healthy behaviors of human development (Shonkoff and Karakowsky, 2014; Pérez-Escamilla et al., 2017). Child development can be altered by complications during pregnancy or childbirth, lack of neurologic stimulation, malnutrition, chronic health problems, and environmental factors (Medina et al., 2015; Gómez-Andrés et al., 2020). ZIKV infection can cause severe brain abnormalities, fetal death, mental retardation, sensorineural deafness (Ventura et al., 2016a; Costello et al., 2017; Schwartz, 2017), and/or eye lesions (de Paula-Freitas et al., 2016; Ventura et al., 2016b). Babies with intrauterine exposure to ZIKV without Congenital Zika Syndrome (CZS) may be at neurodevelopmental risk in the first months of gestation and the first years of life. En México, the impact on the neurological development of children with gestational exposure to the ZIKV has been poorly studied. We aim to describe neurodevelopment in 4–5-year-old infants exposed to ZIKV during pregnancy in Yucatán, México. Materials and MethodsA descriptive cross-sectional study of neurological development was carried out in infants aged 4–5 years, exposed to ZIKV during pregnancy, born at the Regional General Hospital 1 (HGR1) of the Mexican Institute of Social Security (IMSS) in Yucatán, México. The information on maternal gestational conditions, including confirmation of ZIKV infection by RT-qPCR, was retrospectively obtained from the Coordination of Information and Strategic Analysis of the IMSS Yucatán, the Pediatric Service, and the Department of Epidemiology of the HGR1. Clinical and epidemiological data of the mothers who tested positive for ZIKV infection and newborns were obtained from the National Epidemiological Surveillance System and the Epidemiological Control System. Infants with congenital syndromes or congenital infection by agents of the TORCH complex, perinatal asphyxia, and neurological damage from causes other than ZIKV, were excluded. The protocol was approved by the Ethics Committee and the National Committee for Scientific Research of the IMSS with registration number R-2021-785-044. This protocol had no funding. The information on the gestational and postnatal conditions of the infants was obtained from hospital records or provided by the mother, from the WHO growth tables, and the Intergrowth 21st Calculator application (Villar et al., 2013). Height and weight were analyzed using the Body Mass Index (BMI) calculator for children and adolescents. A stimulating environment was considered if infants habitually talked, played, and shared skills and interests with their caregivers. The Child Development Assessment 2nd version (EDI-II) which was developed and validated in México for children under 5 years of age (Rizzoli-Córdoba et al., 2013), was used to classify development as normal, lagged, and at risk of delay with 0.81 sensitivity and 0.61 specificity. The infants with developmental lag or at risk of delay in one or more evaluated areas, performed stimulation exercises according to the altered areas. Later they were reassessed with the EDI-II test and with the Battelle Inventory 2nd version (IDB-II) test. The data were analyzed in frequencies, measures of central tendency, and dispersion with the statistical package SPSS V 25. ResultsMaternal and neonatal characteristics during gestation. A population of 83 pregnant women confirmed with ZIKV infection was treated at HGR1 of the IMSS. Among them, 50 signed the informed consent to the evaluation of their child exposed to the virus during pregnancy. The average maternal age at the time of the infection was 24.78 ± 4.32 years old, and 92% had low obstetric risk due to age. We found that 58% were primiparous, 30% had had one pregnancy, 8% two pregnancies, and 4% three pregnancies. The average gestational age was 18.09 ± 9.22 weeks from the date of last menstruation. ZIKV infection was most frequent in the second gestational trimester (44%) than in the first (34%) and the third (22%) trimester. We found 46 infants (92%) born on the term, 2 infants were pre-term, and 2 post-terms; no data on birth asphyxia were identified. The anthropometric data at birth of all infants were obtained retrospectively from the IMSS hospital records of the somatometry studies made by pediatricians of each infant at birth. We used the WHO growth charts to confirm the measures of the child population at birth. Forty-eight infants (96%) were born with normal weight (3.145 ± 0.375 Kg), 1 case showed 4.650 Kg, and 1 case had 2.290 Kg. Forty-seven neonates (94%) showed normal size (49.54 ± 1.95 cm), 4% and 2% had large and short size, respectively. Most babies (96%) showed normal cephalic perimeter (34.27 ± 1.12 cm), however, when we evaluated the head circumferences in percentiles according to the WHO charts, we identified two newborns with abnormal cephalic perimeter at birth of 32 cm and 39 cm, respectively (Table 1). These two infants had other somatometry results clinically normal at birth as was registered in hospital records. Children’s characteristics at the time of neurodevelopmental assessment. Twenty-nine infants (58%) showed healthy weight (17.07 ± 2.32 Kg), 12 cases (24%) were overweight, 4 cases (8%) were obese, and 5 infants (10%) were underweighted. Most of the child population (90%) showed normal size (103.76 ± 4.58 cm), 8% had height below the 3rd percentile, and 2% had height above the 97th percentile. All children had normal cephalic perimeter (50.7 ± 1.14 cm) including those with a Z Score of 1 to −1 outside normal at birth (Table 1). Neurodevelopmental disorders in infants exposed to ZIKV. We identified 14 children (28%) with developmental lag, and 7 (14%) at risk of developmental delay, with different affectation in different areas (Supplementary material Table S1). The Fine Motor area was the most affected in 12 cases (57%) with lagged development. In the area of knowledge, 43% (9 cases) were found lag and 4.8% (1 case) at risk of being delayed. In Language, a lag was observed in 5 cases (24%), and 3 cases (14.3%) at risk of delay. In the Social area, there were 5 cases (24%) in lag and 1 case at risk of delay. The Gross Motor area was the least affected and all cases (100%) presented a lag in this area. Neurodevelopment alterations by trimester of uterine exposition to ZIKV and gender. Among 12 children with a development lag of the Fine Motor area, 83.3% (10 cases) were male and 58.3% (7 cases) were exposed to ZIKV in the second trimester of pregnancy. The Knowledge was affected in 10 infants, 6 (60% lagging) exposed to ZIKV in the second trimester and 5 cases (50%) male; the only case at risk of delay of Knowledge was a male exposed to ZIKV in the second trimester. Language alterations were observed in 8 infants of which 62.5% had lagging, 60% were exposed to ZIKV in the second trimester, and 80% were male. Among the 3 infants at risk for Language delay, 2 were male and 2 were exposed to ZIKV in the first trimester of pregnancy. Regarding Social development 5 children (83.3%) were classified as lagging, of which 3 cases (60%) were exposed to ZIKV in the second trimester, and 4 cases (80%) were male; the only male at risk of Social delay was exposed to ZIKV in the second trimester of pregnancy. In the Gross Motor area, we observed 100% lagging, half was exposed in the first trimester, and the others in the second; the male sex predominated in 75% of the cases. Regarding the Neurological examination, 1 male exposed to ZIKV in the first trimester of pregnancy presented microcephaly; 2 male infants born pre-term and 2 male post-term had alarm signals. Therefore, these 5 infants were identified as at risk of delay (Table 2). Neurodevelopmental reassessment after a stimulating environment. Eleven children with development lag and 7 children at risk of delay were reassessed after 3 months of stimulation activities at home. After that, 82% of infants with lagging were reclassified as normal and 18% were reclassified at risk of delay. The areas with the greatest alterations were again Fine Motor and Knowledge with 7 cases (38.9%) each, 27.8% (5 cases) had Language impairment, 22.2% (4 cases) showed alteration of the Social area, and 16.7% (3 cases) had alteration of the Gross Motor area. Application of the Battelle Inventory 2nd version (IDB-II). Eight children were evaluated with the IDB-II, the average age was 59 ± 1.2 months, while the equivalent age of the IDB-II was 45 ± 4.5 months of age. The difference indicated an average lag of up to 14 months between chronological age and neurological age. In the Social, Adaptative, and total Motor areas, we obtained the highest differences of up to 22, 23, and 24 months, respectively, in 1 child 57 months old. The Fine Motor area was also the most delayed in the above case and other infants. We observed a difference up to 23 months in the Receptive subarea of another child, and the greatest lag of 30 months in the Expressive component of one case with 59 months old. The Cognitive area had a maximum lag of 16 months in one infant (Table 3). DiscussionSeveral studies carried out in children exposed to ZIKV in utero, with or without microcephaly or congenital infection, reported motor, language, and cognitive abnormalities by different tests at ages 12, 18, 24, 32, and 48 months (Alves et al., 2018; Einspieler et al., 2019; Nielsen-Saines et al., 2019; Vianna et al., 2019; Cranston et al., 2020). We found by first-time neurodevelopment alterations in 4–5-year-old infants born in Yucatán México, exposed to ZIKV during pregnancy. Table 1. Characteristics of the 50 infants at birth and neurodevelopmental assessment.
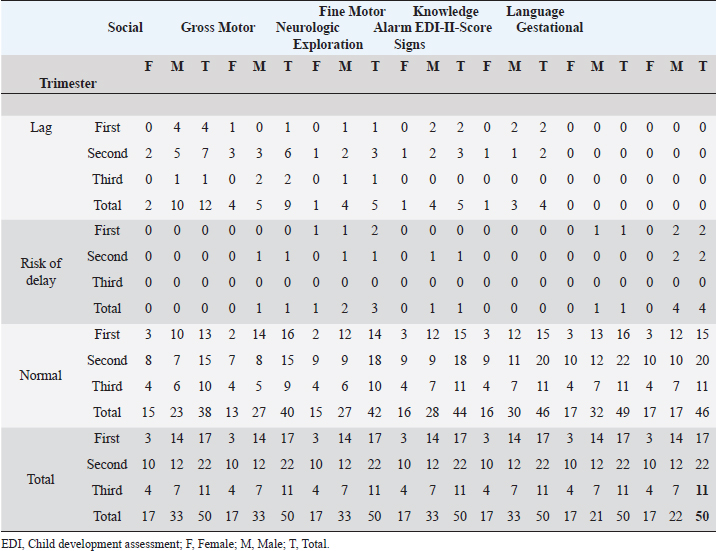
A study of 60 normocephalic and asymptomatic Yucatecan infants about 6 months old, exposed to ZIKV during pregnancy were assessed with the Mullen Scales of Early Learning (MSEL), a test with scales in Motor, Language, and overall Cognitive skills development. All MSEL sub-scale scores except Expressive Language, were significantly lower compared to controls (Familiar et al., 2021). Unfortunately, available medical records for infants did not include information on TORCH, Varicella zoster, or Epstein Barr viruses; thus, it was not discarded as a possible influence of these pathogens. Similarly to our study, the developmental assessment with the EDI-I test of infants 13–24 months after exposition to ZIKV in Oaxaca, México, identified 50% at risk of delay and 33.3% with lag being the Language the main development area affected (Martínez-Pérez et al., 2018). Table 2. Neurodevelopment status by trimester of ZIKV exposition, gender, and area of neurological development.
Table 3. Differences between chronological ages and equivalent ages (IDB-II) in the areas of neurological development.
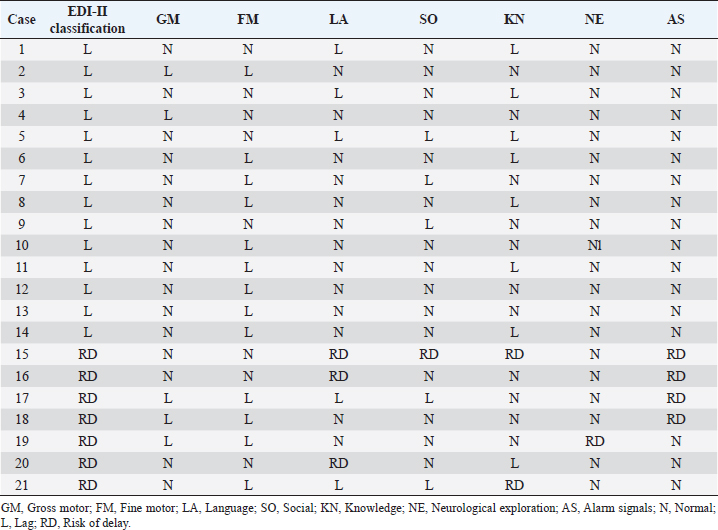
Most of the children evaluated in other studies were exposed to ZIKV in the second trimester. However, we found a higher risk of delay in infants exposed during the first trimester of pregnancy, although we did not observe structural alterations or microcephaly. Similarly, Einspieler et al. (2019) reported fidgety movements in children born to mothers with an acute illness and rash during the first trimester of pregnancy. Vianna et al. (2019) found maternal rash frequency 10 times higher during the first trimester than in the other trimesters (Vianna et al. 2019). Even earlier gestational age at the time of ZIKV infection was a significant predictor of below-average neurodevelopment (Nielsen-Saines et al., 2019). Hoen et al. (2018) reported a 12.7% risk of birth defects when ZIKV exposure occurred in the first trimester, 3.6% in the second, and 5.3% in the third. In addition, a CZS risk of 6.9%, 1.2%, and 0.9% from the first to the third trimester was reported (Hoen et al., 2018). When applying the IDB-ll we observed a 59-month-old patient with a 64-month delay (difference of +5, Table 3) in the Expressive component of the total area of Communication; this means a psychological age greater than their chronological age, which could be explained because, although he was exposed to ZIKV, he had had an adequate stimulating environment by his caregivers. Therefore, we do not rule out the role of ZIKV in the total Communication area gap, however, according to the results of the Expressive subarea in this case, and other areas after neurodevelopmental reassessment of children, we highlighted the importance of stimulation in neurological development in infants exposed to ZIKV during pregnancy, unlike other studies in the same issue. On the other hand, children born at 36 weeks of gestation do not necessarily have a gap in the total area of Communication and they can have a neurological development completely normal unless they have risk factors at birth, as the infant is 36 weeks old with normal somatometry and evolution. In contrast, children born with microcephaly associated with CZS presented neurodevelopmental delay by Denver test up their second year of life, with huge differences between equivalent and chronological ages of Language, Gross Motor, Fine Motor/Adaptive and Personnel/Social areas (Alves et al., 2018). Our results might suggest that the developmental disturbances in children that ZIKV could cause during gestation vary during the first years of life and could be reduced by stimulation acquired in a favorable environment. Given that the maternal and products gestational factors did not influence the alterations in child development in our study, congenital infection could explain the presence of alterations in our population studied, which unfortunately we could not prove due to lack of confirmation of the congenital infection in neonates exposed to ZIKV in Yucatán. We emphasize the importance of performing diagnostic tests on all mother-child pairs with ZIKV exposition during pregnancy, as well as adequate monitoring of the products during the first 5 years of life, to establish the impact of the ZIKV on the neuronal functional of child development. AcknowledgmentThe authors thank Dra. Ana Lavadores May (RIP) for providing data information for the work. Authors´ contributionConception or design of the work, Sánchez-Burgos G.; Acquisition, Formal Analysis, and Interpretation of Data, Sánchez-Burgos G.G. and Acosta-Mass A.G.; Investigation or Methodology, Acosta-Mass A.G., and Calvillo-Cervantes D.E.; Resources, Acosta-Mass, A.G.; Data Curation, Acosta-Mass, A.G., and Calvillo-Cervantes D.E.; Writing – Original Draft Preparation, Sánchez-Burgos, G.G.; Writing – Review & Editing, Sánchez-Burgos G.G. Conflict of interestThe authors declare that there is no conflict of interest. FundingNone. Data availabilityAll data are provided in the manuscript. Any additional data needed can be provided upon reasonable request from the corresponding author. ReferencesAlves, L.V., Paredes, C.E., Silva, G.C., Mello, J.G. and Alves, J.G. 2018. Neurodevelopment of 24 children born in Brazil with congenital Zika syndrome in 2015: a case series study. BMJ Open. 8, e021304. Costello, A., Dua, T., Duran, P., Gülmezoglu, M., Oladapo, O.T., Perea, W., Pires, J., Ramon-Pardo, P., Rollins, N. and Saxena, S. 2016. Defining the syndrome associated with congenital Zika virus infection. Bull. World Health Organ. 94, 406–406A. Cranston, J.S., Tiene, S.F., Nielsen-Saines, K., Vasconcelos, Z., Pone, M.V., Pone, S., Zin, A., Salles, T.S., Pereira, J.P., Jr, Orofino, D., Brasil, P., Kerin, T., Adachi, K., Soares, F.M., Dunshee de Abranches, A., da Costa, A.C.C. and Lopes Moreira, M.E. 2020. Association between antenatal exposure to Zika virus and anatomical and neurodevelopmental abnormalities in children. JAMA Netw. Open. 3, e209303. de Araújo, T.V.B., Rodrigues, L.C., de Alencar Ximenes, R.A., de Barros Miranda-Filho, D., Montarroyos, U.R., de Melo, A.P.L., Valongueiro, S., de Albuquerque, M.F.P.M., Souza, W.V., Braga, C., Filho, S.P.B., Cordeiro, M.T., Vazquez, E., Di Cavalcanti Souza Cruz, D., Henriques, C.M.P., Bezerra, L.C.A., da Silva Castanha, P.M., Dhalia, R., Marques-Júnior, E.T.A., Martelli, C.M.T., investigators from the Microcephaly Epidemic Research Group, Brazilian Ministry of Health, Pan American Health Organization, Instituto de Medicina Integral Professor Fernando Figueira, and State Health Department of Pernambuco. 2016. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect. Dis. 16, 1356–1363. de Oliveira, W.K., de França, G.V.A., Carmo, E.H., Duncan, B.B., de Souza Kuchenbecker, R. and Schmidt, M.I. 2017. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet 390, 861–870. de Paula-Freitas, B., de Oliveira-Dias, J.R., Prazeres, J., Sacramento, G.A., Ko, A.I., Maia, M. and Belfort, R. Jr. 2016. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. 134, 529–535. Einspieler, C., Utsch, F., Brasil, P., Panvequio Aizawa, C.Y., Peyton, C., Hydee Hasue, R., Françoso Genovesi, F., Damasceno, L., Moreira, M.E., Adachi, K., Marschik, P.B., Nielsen-Saines, K. and GM Zika Working Group. 2019. Association of infants exposed to prenatal Zika virus infection with their clinical, neurologic, and developmental status evaluated via the general movement assessment tool. JAMA Netw. Open. 2, e187235. Familiar, I., Boivin, M., Magen, J., Azcorra, J.A., Phippen, C., Barrett, E.A., Miller, S. and Ruisenor-Escudero, H. 2021. Neurodevelopment outcomes in infants born to women with Zika virus infection during pregnancy in Mexico. Child Care Health Dev. 47, 311–318. Gómez-Andrés, D., Pulido Valdeolivas, I. and Fiz Pérez, L. 2020. Desarrollo neurológico normal del niño. Pediatriaintegral.es. Available via https://www.pediatriaintegral.es/wp-content/uploads/2015/xix09/07/n9-640e1-e7_R.Bases_Gomez.pdf Hernández-Ávila, J.E., Palacio-Mejía, L.S., López-Gatell, H., Alpuche-Aranda, C.M., Molina-Vélez, D., González-González, L. and Hernández-Ávila, M. 2018. Zika virus infection estimates, Mexico. Bull. World Health Organ. 96, 306–313. Hoen, B., Schaub, B., Funk, A.L., Ardillon, V., Boullard, M. and Cabié, A. 2018. Pregnancy outcomes after ZIKV infection in French territories en the Americas. N. Eng. J. Med. 378, 985–994. Martínez-Pérez, M.I., Cervantes-Quiróz, A.R., Cacique-Sánchez, C., Gómez-Bautista, J.B., Hernández-Peláez, D.K. and Matías-González, A. 2018. Alteraciones del desarrollo neurológico en productos con infección gestacional por virus Zika. Avan. C. Salud Med. 5, 120–128. Medina, A.M.P., Caro Kahn, I., Muñoz Huerta, P., Leyva Sánchez, J., Moreno Calixto, J. and Vega Sánchez, S.M. 2015. Neurodesarrollo infantil: características normales y signos de alarma en el niño menor de cinco años. Rev. Peru. Med. Exp. Salud Pública. 32, 565–573. Nielsen-Saines, K., Brasil, P., Kerin, T., Vasconcelos, Z., Gabaglia, C.R., Damasceno, L., Pone, M., Abreu de Carvalho, L.M., Pone, S.M., Zin, A.A., Tsui, I., Salles, T.R.S., da Cunha, D.C., Costa, R.P., Malacarne, J., Reis, A.B., Hasue, R.H., Aizawa, C.Y.P., Genovesi, F.F., Einspieler, C., Marschik, P.B., Pereira, J.P., Gaw, S.L., Adachi, K., Cherry, J.D., Xu, Z., Cheng. G. and Moreira, M.E. 2019. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 25, 1213–1217. Pérez-Escamilla, R., Rizzoli-Córdoba, A., Alonso-Cuevas, A. and Reyes-Morales, H. 2017. Advances in early childhood development: from neurons to big scale programs. Bol. Med. Hosp. Infant. Mex. 74, 86–97. Rizzoli-Córdoba, A., Schnaas-Arrieta, L., Liendo-Vallejos, S., Buenrostro-Márquez, G., Romo-Pardo. B. and Carreón-García, J. 2013. Validación de un instrumento para la detección oportuna de problemas de desarrollo en menores de 5 años en México. Bol. Med. Hosp. Infant. Mex. 70, 195–208. Schwartz, D.A. 2017. Autopsy and postmortem studies are concordant: pathology of Zika virus infection is neurotropic in fetuses and infants with microcephaly following transplacental transmission. Arch. Pathol. Lab. Med. 141, 68–72. Secretaría de Salud, Dirección General de Epidemiología, Subsecretaría de Prevención y Promoción de la Salud. 2021. Casos Confirmados Autóctonos de Enfermedad por Virus del Zika por Entidad Federativa. Gob. Mex. Bull. 52, 1-5. Available via: https://www.gob.mx/cms/uploads/attachment/file/691598/CuadroCasosZikayEmbsem52_2021.pdf Shonkoff, J.P. and Karakowsky, Y. 2014. Neurobiología del desarrollo en la primera infancia. Fundación para una sociedad sostenible. In Los invisibles: Las niñas y los niños de 0 a 6 años. Eds., Santibañez-Martínez, L., Calderón Martín del Campo, D.E. and México, D.F, pp: 23–46. Ventura, C.V., Maia, M., Bravo-Filho, V., Góis, A.L. and Belfort, R. Jr. 2016a. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 387, 228. Ventura, C.V., Maia, M., Ventura, B.V., Linden, V.V., Araújo, E.B., Ramos, R.C., Rocha, M.A., Carvalho, M.D., Belfort, R. Jr. and Ventura, L.O. 2016b. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq. Bras. Oftalmol. 79, 1–3. Vianna, R.A.O., Lovero, K.L., Oliveira, S.A., Fernandes, A.R., Santos, T.C.S.D., Lima, L.C.S.S., Carvalho, F.R., Quintans, M.D.S., Bueno, A.C., Torbey, A.F.M., Souza, A.L.A.A.G., Farias, A.O.P., Camacho, L.A.B., Riley, L.W. and Cardoso, C.A.A. 2019. Children born to mothers with rash during Zika virus epidemic in Brazil: first 18 months of life. J. Trop. Pediatr. 65, 592–602. Villar, J., Altman, D.G., Purwar, M., Noble, J.A., Knight, H.E., Ruyan, P., Cheikh Ismail, L., Barros, F.C., Lambert, A., Papageorghiou, A.T., Carvalho, M., Jaffer, Y.A., Bertino, E., Gravett, M.G., Bhutta, Z.A., Kennedy. S.H. and International Fetal and Newborn Growth Consortium for the 21st Century. 2013. The objectives, design, and implementation of the INTERGROWTH-21st project. BJOG 120 Suppl 2, 9–26. Supplementary MaterialSupplementary Table S1. Neurological development from the child population studied with lag or risk of delay.
| ||
| How to Cite this Article |
| Pubmed Style Acosta-mass AG, Calvillo-cervantes DE, Sánchez-burgos GG. Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations. J Microbiol Infect Dis. 2024; 14(2): 38-45. doi:10.5455/JMID.2024.v14.i2.1 Web Style Acosta-mass AG, Calvillo-cervantes DE, Sánchez-burgos GG. Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations. https://www.jmidonline.org/?mno=190699 [Access: January 25, 2026]. doi:10.5455/JMID.2024.v14.i2.1 AMA (American Medical Association) Style Acosta-mass AG, Calvillo-cervantes DE, Sánchez-burgos GG. Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations. J Microbiol Infect Dis. 2024; 14(2): 38-45. doi:10.5455/JMID.2024.v14.i2.1 Vancouver/ICMJE Style Acosta-mass AG, Calvillo-cervantes DE, Sánchez-burgos GG. Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations. J Microbiol Infect Dis. (2024), [cited January 25, 2026]; 14(2): 38-45. doi:10.5455/JMID.2024.v14.i2.1 Harvard Style Acosta-mass, A. G., Calvillo-cervantes, . D. E. & Sánchez-burgos, . G. G. (2024) Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations. J Microbiol Infect Dis, 14 (2), 38-45. doi:10.5455/JMID.2024.v14.i2.1 Turabian Style Acosta-mass, Arlene G., David E. Calvillo-cervantes, and Gilma G. Sánchez-burgos. 2024. Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations. Journal of Microbiology and Infectious Diseases, 14 (2), 38-45. doi:10.5455/JMID.2024.v14.i2.1 Chicago Style Acosta-mass, Arlene G., David E. Calvillo-cervantes, and Gilma G. Sánchez-burgos. "Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations." Journal of Microbiology and Infectious Diseases 14 (2024), 38-45. doi:10.5455/JMID.2024.v14.i2.1 MLA (The Modern Language Association) Style Acosta-mass, Arlene G., David E. Calvillo-cervantes, and Gilma G. Sánchez-burgos. "Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations." Journal of Microbiology and Infectious Diseases 14.2 (2024), 38-45. Print. doi:10.5455/JMID.2024.v14.i2.1 APA (American Psychological Association) Style Acosta-mass, A. G., Calvillo-cervantes, . D. E. & Sánchez-burgos, . G. G. (2024) Infants with 4-5 years after intrauterine exposition to zika virus have risk of neurodevelopmental alterations. Journal of Microbiology and Infectious Diseases, 14 (2), 38-45. doi:10.5455/JMID.2024.v14.i2.1 |