| Research Article Online Published: 27 Dec 2024 | ||
J Microbiol Infect Dis. 2024; 14(4): 186-190 J. Microbiol. Infect. Dis., (2024), Vol. 14(4): 186–190 Research Article Experience with improved version of cartridge-based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosisDivjot Singh Chawla, Jyoti Chaudhary*, Veenu Gupta, Akashdeep singh, Manisha AggarwalDepartment of Microbiology, Dayanand Medical College and Hospital Ludhiana, Baba Farid University of Health Sciences Faridkot, 141001, Punjab, India *Corresponding Author: Jyoti Chaudhary, Professor Department of Microbiology Dayanand Medical College and Hospital,Ludhiana, Punjab, India. Email: drjyotisohu [at] gmail.com Submitted: 12/09/2024 Accepted: 16/12/2024 Published: 31/12/2024 © 2024 Journal of Microbiology and Infectious Diseases
ABSTRACTBackground: Abdominal TB includes peritoneal and gastrointestinal TB. Diagnosis of extrapulmonary tuberculosis, including abdominal tuberculosis (TB), always remained challenging, thus leading to missed and delayed diagnosis. Point-of-care test such as CBNAAT may help in early diagnosis. Aim: To evaluate the performance of CBNAAT (GeneXpert Ultra) in peritoneal and abdominal lymph node tuberculosis. Material and methods: The prospective study was conducted in the Department of Microbiology after the ethical committee’s approval. All specimens with a requisition for CBNAAT ultra from clinically suspected admitted patients of abdominal TB (tuberculosis), were included. The specimens were subjected to microscopy (ZN stain), culture, and CBNAAT (Xpert MTB/RIF assay Ultra) and processed as per standard protocols. The data were analyzed statistically. Results: Among 331 suspected gastrointestinal TB patients, 65.3% were males, and the maximum number of patients (33.80%) belonged to the age of more than 60 years. The most common presenting symptom was fever (98.8%), followed by abdominal discomfort (84.5%). Comorbidities such as hypertension (56.5%) and diabetes mellitus (55.6%) were observed. ADA (Adenosine Deaminase > 39 U/L) was raised in 20.5% of patients. Out of the total samples tested, 86% were ascitic fluid, 10.9% were lymph nodes, and 3% were pus samples. Among these, CBNAAT was positive in 14 samples, and LJ (Lowenstein Jensen) culture in 6 samples, and none was AFB smear positive. Out of 331 patients, 34 met the criteria of composite reference standard (CRS). On comparison with culture, CBNAAT showed sensitivity, specificity, PPV, and NPV of 100%, 97.54%, 42.86%, and 100%, respectively. While considering CRS as the gold standard CBNAAT showed a sensitivity of 38.24%, specificity of 99.66%, PPV of 92.86%, and NPV of 93.38%. Conclusion: CBNAAT ultra-detected cases that have been missed by other diagnostic methods and increased the diagnostic yield. Still, low sensitivity was observed with CRS criteria. Hence, a combined clinical, radiological, laboratory, and treatment response is the best approach to diagnose the disease. Keywords: Xpert MTB/RIF Ultra assay, abdominal tuberculosis, sensitivity, specificity, composite reference standard INTRODUCTIONTuberculosis (TB) is a major health problem, particularly among immunocompromised patients, the second leading infectious killer after COVID-19, worldwide. It is estimated that one-third of the world’s population is infected with TB. The World Health Organization declared it a global emergency (Mekkaoui et al. 2021). In 2021, an estimated 10.6 million people fell ill with TB worldwide and the major global TB burden (56%) is represented by the South East Asia and Western Pacific regions. Among these, India itself accounts for one-fourth of the cases (WHO 2014). The primary site of TB is usually the lung, it can disseminate to other parts of the body and can affect any organ (Mukewar et al. 2012). Extrapulmonary tuberculosis accounts for 20% of tuberculosis patients (Pai, 2014). Abdominal TB is one of the most common forms of extra-pulmonary TB and constitutes 12% of all extra-pulmonary TB cases (Sheer 2003), Abdominal TB includes the involvement of the peritoneum with ascites or lymph nodes or any intra-abdominal organ. The infection may occur due to swallowing the infected sputum, ingestion of unpasteurized milk (Mycobacterium bovis), or by hematogenous spread. Patients usually present with fever and abdominal pain most frequently, with small bowel obstruction or gastrointestinal bleeding. The liver, spleen, pancreas, and adrenal glands may also be involved (Thwaites et al. 2009). The diagnosis of extra-pulmonary TB is difficult as it presents nonspecific clinical and radiological features (WHO 2014). Moreover, there is a considerable diagnostic dilemma for abdominal TB, as it can mimic other diseases such as Crohn’s disease, abdominal malignancies, or colitis, also called the great mimicker (Shreshtha et al. 2016). Other laboratory tests such as high ADA, raised ESR, and lymphocytosis supports the diagnosis. Among the microbiological tests, the culture of M. tuberculosis is considered the gold standard. However, it is a lengthy procedure with 2-6 weeks of turnaround time and the paucibacillary nature of the bacteria makes it difficult to detect, so the risk of false negatives is very likely (Chander, 2013). Therefore, microscopy and molecular methods such as CBNAAT/TRUENAT are recommended as tests of choice for primary diagnosis. Microscopy has very low sensitivity in extrapulmonary samples. These PCR-based tests are rapid and provide results within a few hours with high sensitivity and specificity and simultaneously detect drug resistance. CBNAAT ultra has been introduced with higher sensitivity and a lesser turnaround time than CBNAAT. Though molecular tests have revolutionized the diagnosis and treatment of EPTB, many times diagnosis is based upon clinical, radiological, histopathology, and other laboratory tests (Zidan et al. 2019). Varied sensitivity and specificity of CBNAAT have been reported in extrapulmonary samples by the studies. Very few studies are available on CBNAAT ultra. Hence the study was planned to evaluate the role of CBNAAT ultra using culture & clinical CRS criteria. MATERIAL AND METHODSThis prospective study was conducted in the Department of Microbiology, Dayanand Medical College and Hospital, Ludhiana. The study was approved by the ethical committee of the institute. Inclusion CriteriaAll specimens with a requisition for CBNAAT from clinically suspected patients of abdominal TB, admitted to various wards and the intensive care units of the hospital were included. Exclusion CriteriaSample mixed with blood, insufficient sample (<1 mL), and outdoor samples were excluded. Demographic and clinical details such as age, sex, clinical presentation, underlying diseases, laboratory parameters, diagnostic methods, and anti-tubercular treatment were noted. Specimen ProcessingThe specimens were processed in the Microbiology laboratory as per standard protocols. Sterile fluids were processed without decontamination and non-sterile specimens such as pus and tissue samples were decontaminated using N-Acetyl-L-Cysteine method. Tissue was homogenized with sterile water or saline (2–4 mL) in a pre-sterilized tissue grinder. Pus samples that were thick or mucoid were liquefied prior to centrifugation by adding NACL powder (50–100 mL). After centrifugation, the sediment was re-suspended in about 5 mL of saline and then decontaminated, and body fluids were processed without decontamination. It was concentrated by centrifugation at 3000 rpm for 15–20 mins (Collee et al. 1996). Each specimen after centrifugation was subjected to:
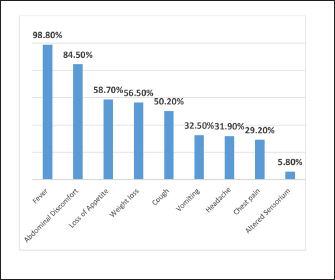
Sample processing for GeneXpert MTB/RIF Ultra (Cepheid, Sunnyvale, CA): The fluids (10–15 mL) were centrifuged, the pellet was suspended in 0.5 mL normal saline, and tissues (minimum ½ inch) were washed if blood tinged and homogenized. Buffer (2 mL) was added to the processed samples and incubated at room temperature for 15 min with two vortex steps. Pipette 2 mL of it and load slowly into the S‑chamber of the cartridge carefully without introducing any bubbles. The cartridge was loaded into the machine after adding the sample as per the manufacturer’s guidelines. Composite Reference Standard (CRS)The CRS criteria included patients suspected for TB and positive for TB culture or CBNAAT and patients with negative/trace CBNAAT results along with clinical, laboratory, or radiological findings suggestive of TB and response to ATT. Cases either with an alternate diagnosis or not fit into CRS criteria were considered “Non-TB” Vadwai et al. 2011). Statistical AnalysisAll the data were entered in Microsoft Excel and the statistical analysis was performed using Epi-data analysis software (version V2.2.2.178). Data were calculated on the basis of appropriate statistical analysis. The sensitivity and specificity of Xpert Ultra were calculated by comparing it with culture and composite reference standard (CRS). RESULTSAmong 331 suspected gastrointestinal patients, the majority of patients (33.8%) belonged to more than 60 years age group, followed by (19.6%) in 51–60 years of age group, (19.3%) in 41–50 years of age group, (13%) in 31–40 years of age group, (7.8%) in 21–30 years of age group, (4.3%) of 10–20 years, and (1.8%) in less than 10 years of age group. Out of 331 patients, 65.2% (216) were male patients and 34.7% (115) were females. The maximum number of samples were ascitic fluid 86.1% (285) followed by lymph node and pus samples 13.8% (46). The most common presenting symptom was fever (327) 98.8%, followed by abdominal discomfort (279) 84.5%, loss of appetite (194) 58.7%, and weight loss (187) 56.5% (Figure 1). The most common comorbidity was hypertension 56.5% followed by diabetes mellitus 55.6%.
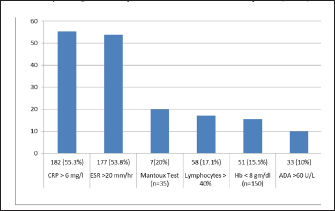
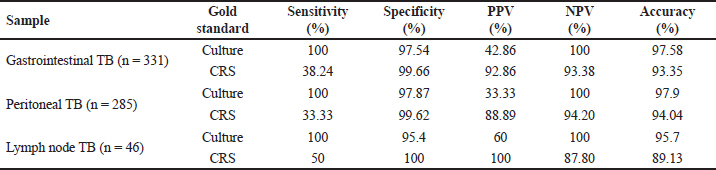
Fig. 1. Distribution according to clinical symptoms of the patients (n=331). Among the enrolled patients, 55.3% (182) had raised CRP (>6 mg/mL), 53.8% (177) had raised ESR (>20 mm/hour), 15.5% (51) presented with severe anemia (<8 mg/dL), while 17.7% (58) had lymphocytosis (>40% lymphocytes). ADA was raised (>39 U/L ) in 21.1% of patients and taking criteria as (>60 U/L) was seen in 10.0% of patients (Figure 2). Mantoux test was performed on 35 patients and 20% of patients showed a positive reaction. Radiological abnormalities were noted in 23 (6.9%) patients. The most common presentation in radiology was free fluid in the abdomen, patchy consolidation, and nodular lesions in the liver. Out of 331 suspected gastrointestinal patients, Xpert Ultra was positive in 14 samples (M. tuberculosis detected medium in only in 1, low in 8, trace in 3, and very low in 2 samples), culture in 6 samples (LJ culture in 5 and MGIT in 1), and none of the sample was positive for AFB smear. Among the CBNAAT-positive isolates, none of the samples showed rifampicin resistance. Out of 331 patients, 34 patients met the criteria of CRS, including 6 confirmed cases and 28 possible/probable cases. For gastrointestinal tuberculosis, in comparison with culture, Xpert Ultra showed sensitivity, specificity, PPV, and NPV of 100%, 97.54%, 42.86%, and 100%, respectively. While considering CRS as the gold standard, CBNAAT showed a sensitivity of 38.24%, specificity of 99.66%, PPV of 92.86%, and NPV of 93.38% (Table 1). DISCUSSIONDiagnosis of EPTB has always remained challenging over decades, particularly in cases of tubercular ascites due to its paucibacillary nature, thus leading to missed and delayed diagnosis (Chander et al. 2013). Newly introduced NAATs (Nucleic Acid Amplification Tests), if used in combination with other tests provide improved diagnostic yield and are now being widely used (Maynard-Smith et al. 2014). In our study, out of a 331 enrolled patients, CBNAAT was positive in 14 patients (4.2%), and culture was positive in 6 (1.8%) patients, whereas a study conducted by (Rufai et al. 2017) showed positivity (17.9%) of CBNAAT and culture (25.4%), and Ahmad et al. (2018) showed 28.6% CBNAAT positivity. In our study, AFB smear of all samples were negative, similar results were reported in another study (Rufai et al 2017). The majority of the patients in our study were more than 60 years (34%) and the mean age group was 49.3 years, while in a study by (Dhali et al. 2021) younger age group was affected with mean age of 45 years, and in a study by (Ahmad et al. 2018) predominant age group was less than 30 years (76.2%). Male patients were affected more (65.2%) in our study as compared to females (34.7%). These results alike to a study by (Rufai et al. 2017) who reported 64.2% of males who had TB (Rufai 1. 2017). Intestinal tuberculosis vary clinically, this makes it difficult to distinguish intestinal tuberculosis from other intestinal diseases. Abdominal pain is chronic but can also be acute on chronic if acute complications occur. Weight loss is also the most common symptom that occurs in patients with intestinal tuberculosis due to various causes such as chronic inflammatory processes, decreased intake, and impaired absorption. Weight loss can be accompanied by mild to moderate anemia. Mostly intestinal tuberculosis patients experience irregular low-grade fever, with a body temperature between 37.5 and 38.5°C, followed by night sweats. In our study, fever was the most common presentation (98.8%), followed by abdominal pain (84.5%), loss of appetite (58.7%), and weight loss in (56.5%) of patients. While in a study by (Patel et al 2018) abdominal pain (95.5%) was the most common symptom, followed by fever (80.6%), whereas a study by (Ahmad et al. 2018) reported weight loss and fever in 47.6% of patients and abdominal pain in 38.1% of patients Ascitic fluid ADA levels are elevated in tubercular ascites. Serum ADA level above 54 U/L, ascitic fluid ADA level above 36 U/L, and an ascitic fluid to serum ADA ratio of more than 0.98 are suggestive of tuberculosis. In our study, taking >60 U/L as criteria, it was raised in 33 samples (10.1%); however, considering Index TB guidelines (ADA > 39 U/L as criteria), it was increased in 68 samples (20.5%), results were similar to the results of other authors (Dhali et al. 2021). The various radiological studies used to diagnose abdominal TB include ultrasonography (USG), CT, barium studies, and magnetic resonance imaging (MRI). Ultrasound is an initial modality of choice that is useful in picking up lymphadenopathy, tubercular ascites, peritoneal thickening, omental thickening, and bowel wall thickening in some cases. Radiological abnormalities in our study were observed in 23 (6.9%) patients. The most common presentation was free fluid in the abdomen with enlarged mesenteric lymph nodes and nodular lesions in the liver.
Fig. 2. Laboratory investigations of suspected Gastrointestinal tuberculosis patients (n=331). GeneXpert assay is a fully automated real-time PCR-based test used for the detection of mutations related to the resistance of M. tuberculosis to rifampicin. The sensitivity and specificity of this investigation are quite high and the results can be obtained in a short time (<2 hours). So this is quite recommended to be performed in patients with suspected intestinal tuberculosis. In our study, sensitivity and specificity of CBNAAT for peritoneal TB in comparison to culture were 100% and 97.54%, respectively, whereas other studies reported a sensitivity of 70.58% and a specificity of 100%. (Rufai et al. 2017). In our study, overall sensitivity, specificity, and accuracy of CBNAAT in comparison to CRS criteria were 38.24%, 99.66 %, and 93.35%, respectively. Kumar et al. (2017) reported the sensitivity, specificity, positive predictive value, and negative predictive value of the Xpert MTB/RIF assay were 8.1%, 100%, 100%, and 64.2%, respectively (Kumar S et al. 2017). Like our study, they did not report MDR abdominal tuberculosis cases in their study. Table 1. Sample-wise Sensitivity, Specificity, PPV, NPV and Accuracy of CBNAAT compared with Culture and CRS.
The pooled sensitivity and specificity with respect to culture in a study by (Kohli et al 2021) were 64% and 97% and with CRS were 30% and 100%. Our study showed 100% sensitivity of CBNAAT with culture. CRS, usually diagnose TB on the basis of clinical features, laboratory reports, might confirm the positivity of Xpert MTB/RIF for a sample with negative TB culture (Sharma et al. 2021). In a study by Kohli et al. (2021), the pooled sensitivity and specificity with respect to culture were 64% and 97% and with CRS was 30% and 100%, mostly results were in concordance with our study with better sensitivity of CBNAAT 100% with culture. CRS, which usually diagnoses TB on the basis of clinical features, laboratory reports, might confirm the positivity of Xpert MTB/RIF for a sample with negative TB culture and can enhance Xpert MTB/RIF‘s accuracy (Sharma et al. 2021). Out of a total of 331 patients, 34 (10.2%) patients fulfilled the CRS criteria for TB. Out of them, 6 were confirmed, 10 were possible, and 18 probable cases. Of the CRS-positive patients, 82.4% (28/34) were culture negative, which was attributed to the low sensitivity of culture. The low sensitivity of culture could be explicated by the fact that patients can be receiving anti-tuberculosis treatment (ATT) or other possible explanations include the paucibacillary nature of extra-pulmonary specimens and loss of viable bacilli during the NALC–NaOH decontamination procedure. In conclusion, early diagnosis is key for the successful treatment and control of TB. Xpert Ultra has increased the confirmatory diagnosis of EPTB. Clinical, endoscopic, radiological, cytology, and microbiological findings collectively are the best approach to diagnosing abdominal TB. FUNDINGThesis Grant by state TB cell, NTEP Government of Punjab. ACKNOWLEDGMENTSSWe acknowledge State Task Force, NTEP, Government of Punjab. CONFLICT OF INTERESTAll authors declare no competing interests. AUTHORS’ CONTRIBUTIONAll the authors contributed equally to the article DATA AVAILABILITY STATEMENTThe data can be obtained from the corresponding author on a reasonable request. STATEMENT OF INFORMED CONSENTConsent was obtained from the enrolled patients . REFERENCESAhmad, R., Changeez, M., Khan, J.S., Qureshi, U., Tariq, M., Malik, S., Ahmad, S.H., Shafique, M.S. 2018. Diagnostic accuracy of peritoneal fluid GeneXpert in the diagnosis of intestinal tuberculosis, keeping histopathology as the gold standard. Cureus. 10(10):e3451. doi: 10.7759/cureus.3451. PMID: 30564530; PMCID: PMC6298629. Chander, A. 2013. Diagnostic significance of ascites adenosine deaminase levels in suspected tuberculous peritonitis in adults. J Microbiol Infect Dis. 3(3):104–8. Collee, J.G., Miles, R.S. and Watt, B. (1996) Tests for the Identification of Bacteria. In: Collee, J.G., Marmion, B.P., Fraser, A.G. and Simmons, A., Eds., Mackie & McCartney Practical Medical Microbiology, 14th Edition, Churchill Livingstone, New York, 131–151. Dhali, A., Das, K., Dhali, G.K., Ghosh, R., Sarkar, A., Misra, D. 2021. Abdominal tuberculosis: clinical profile and outcome. Int J Mycobacteriol. 10(4):414–20. doi: 10.4103/ijmy.ijmy_195_21. PMID: 34916461. Kohli, M., Schiller, I., Dendukuri, N., et al. 2021. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 1(1): CD012768. Kumar, S., Bopanna, S., Kedia, S., et al. 2017. Evaluation of Xpert MTB/RIF assay performance in the diagnosis of abdominal tuberculosis. Intest Res. 15(2):187–194. doi:10.5217/ir.2017.15.2.18 Maynard-Smith L, Larke N, Peters JA, Lawn SD. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis. 2014 Dec 31;14:709. doi: 10.1186/s12879-014-0709-7. PMID: 25599808; PMCID: PMC4298952. Mekkaoui, L., Hallin, M., Mouchet, F., Payen, M.C., Maillart, E., Clevenbergh, P., et al. 2021. Performance of Xpert MTB/RIF Ultra for diagnosis of pulmonary and extra-pulmonary tuberculosis, one year of use in a multi-centric hospital laboratory in Brussels, Belgium. PLoS One. 16(4):0249734. Mukewar, S., Mukewar, S., Ravi, R., Prasad, A, S Dua K. 2012. Colon tuberculosis: endoscopic features and prospective endoscopic follow-up after anti-tuberculosis treatment. Clin Transl Gastroenterol. 3(10):e24. [PMID: 23238066 DOI: 10.1038/ ctg.2012.19] Pai, M., Nathavitharana, R. 2014. Extrapulmonary tuberculosis: new diagnostics and new policies. Indian J Chest Dis Allied Sci. 56(2):71–3. Patel B, Yagnik VD. Clinical and laboratory features of intestinal tuberculosis. Clin Exp Gastroenterol. 2018 Mar 13;11:97-103. doi: 10.2147/CEG.S154235. PMID: 29559804; PMCID: PMC5856297. Rufai, S.B., Singh, S., Singh, A., Kumar, P., Singh, J., Vishal, A. 2017. Performance of Xpert MTB/RIF on ascitic fluid samples for detection of abdominal tuberculosis. J Lab Physicians. 9(1):47–52. doi: 10.4103/0974-2727.187927. PMID: 28042217; PMCID: PMC5015498. Sharma, V., Soni, H., Kumar-M. P., Dawra, S., Mishra, S., Mandavdhare, H.S., Singh, H., Dutta, U. 2021. Diagnostic accuracy of the Xpert MTB/RIF assay for abdominal tuberculosis: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 19(2):253–65. doi: 10.1080/14787210.2020.1816169. Epub 2020 Sep 20. PMID: 32845790 Sheer, T.A., Coyle, W.J. 2003. Gastrointestinal tuberculosis. Curr Gastroenterol Rep. 5(4):273–8. Shreshtha, S., Ghuliani, D. 2016. Abdominal tuberculosis: a retrospective analysis of 45 cases. Indian J Tuberc. 63(4):219–24. Thwaites, G., Fisher, M., Hemingway, C., Scott, G., Solomon, T., Innes, J., et al. 2009. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J infect. 59(3):167–87. Vadwai, V., Boehme, C., Nabeta, P., Shetty, A., Alland D. and Rodrigues, C. 2011. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin. Microbiol. 49 (7): 2540–45. World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; 2014. 12;14:33. doi: 10.1186/s13017-019-0252-3. Erratum in: World J Emerg Surg. 2019 Aug 16;14:42. doi: 10.1186/s13017-019-0260-3. PMID: 31338118; PMCID: PMC6626328. | ||
| How to Cite this Article |
| Pubmed Style Chawla DS, Chaudhary J, Gupta V, Singh A, Aggarwal M. Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis. J Microbiol Infect Dis. 2024; 14(4): 186-190. doi:10.5455/JMID.20240912084953 Web Style Chawla DS, Chaudhary J, Gupta V, Singh A, Aggarwal M. Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis. https://www.jmidonline.org/?mno=220118 [Access: January 08, 2026]. doi:10.5455/JMID.20240912084953 AMA (American Medical Association) Style Chawla DS, Chaudhary J, Gupta V, Singh A, Aggarwal M. Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis. J Microbiol Infect Dis. 2024; 14(4): 186-190. doi:10.5455/JMID.20240912084953 Vancouver/ICMJE Style Chawla DS, Chaudhary J, Gupta V, Singh A, Aggarwal M. Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis. J Microbiol Infect Dis. (2024), [cited January 08, 2026]; 14(4): 186-190. doi:10.5455/JMID.20240912084953 Harvard Style Chawla, D. S., Chaudhary, . J., Gupta, . V., Singh, . A. & Aggarwal, . M. (2024) Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis. J Microbiol Infect Dis, 14 (4), 186-190. doi:10.5455/JMID.20240912084953 Turabian Style Chawla, Divjot Singh, Jyoti Chaudhary, Veenu Gupta, Akashdeep Singh, and Manisha Aggarwal. 2024. Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis. Journal of Microbiology and Infectious Diseases, 14 (4), 186-190. doi:10.5455/JMID.20240912084953 Chicago Style Chawla, Divjot Singh, Jyoti Chaudhary, Veenu Gupta, Akashdeep Singh, and Manisha Aggarwal. "Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis." Journal of Microbiology and Infectious Diseases 14 (2024), 186-190. doi:10.5455/JMID.20240912084953 MLA (The Modern Language Association) Style Chawla, Divjot Singh, Jyoti Chaudhary, Veenu Gupta, Akashdeep Singh, and Manisha Aggarwal. "Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis." Journal of Microbiology and Infectious Diseases 14.4 (2024), 186-190. Print. doi:10.5455/JMID.20240912084953 APA (American Psychological Association) Style Chawla, D. S., Chaudhary, . J., Gupta, . V., Singh, . A. & Aggarwal, . M. (2024) Experience with improved version of cartridge based nucleic acid amplification test (GeneXpert Ultra) in abdominal tuberculosis. Journal of Microbiology and Infectious Diseases, 14 (4), 186-190. doi:10.5455/JMID.20240912084953 |