| Review Article | ||
J. Microbiol. Infect. Dis., (2025), Vol. 15(1): 5–18 Review Article Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implicationsAminath Shafeenaz Moosa1, Aishath Zeena Abdul Jaleel1, Shifa Ishaq1, Saifulla Muslim1, Suha Ibraheem1, Mariyam Niusha Naseer1, Kannan Subbaram1*, Zeba Un Naher1, Razana Faiz1, Aminath Huda1, Punya Laxmi Manandhar1, Sheeza Ali1, Sina Salajegheh Tazerji2 and Phelipe Magalhães Duarte31School of Medicine, The Maldives National University, Male’, Maldives 2Faculty of Veterinary Medicine, Science and Research Branch, Islamic Azad University, Tehran, Iran 3Postgraduate Program in Animal Bioscience, Federal Rural University of Pernambuco (UFRPE), Recife, Brazil *Corresponding Author: Kannan Subbaram. School of Medicine, The Maldives National University, Male’, Maldives. Email: kannan.subbaram [at] mnu.edu.mv Submitted: 08/03/2024 Accepted: 09/02/2025, Published: 31/03/2025 © 2025 Journal of Microbiology and Infectious Diseases
ABSTRACTAntimicrobial resistance (AMR) is a serious public health issue and leads to a severe warning to clinicians, public healthcare specialists, and healthcare system. There are a variety of bacteria, viruses, fungi, protozoa, and helminths that have acquired AMR. This article aims to bring the recent update on AMR in bacteria, its global prevalence, morbidity and mortality, mechanisms, implications, and consequences. A detailed study was conducted using Google Scholar, MEDLINE/PubMed, NCBI, EBSCO, ScienceDirect, SCOPUS, and Web of Science data bases. All the concerned areas of research on AMR were retrieved. Important bacteria that have emerged as multiple antibiotic resistance are, Staphylococcus aureus (S. aureus), Neisseria gonorrhoea, Enterococcus, Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli), Mycobacterium tuberculosis (M. tuberculosis), and Acinetobacter baumannii (A. baumannii). Recently there were many cases of vancomycin resistance S. aureus as well as vancomycin resistance Enterococcus were reported from many parts of the world. Due to antibiotic abuse/misuse of antibiotic therapy, several bacteria acquired multiple antibiotic resistance. The diseases produced due to these pathogens are hard to manage or cannot be treated at all. In the future, the AMR in bacteria poses a severe threat to entire humanity due to increased mortality and morbidity. National and international health agencies should take urgent and immediate action to address this issue. Keywords: Antimicrobial resistance, AMR, Antibiotics, Silent pandemic, Bacteria. IntroductionAntimicrobial agents, which are extensively used in medicine, include antibacterial agents, antifungals, antiparasitic, and antiviral agents. These antimicrobials are used to treat diseases in plants, animals, and humans. Resistance against antibiotics/antimicrobial resistance (AMR) develops during the evolution of pathogens, and then they acquire resistance against medications (Pan et al., 2024). The microorganisms acquire the capacity to overcome the action of antimicrobials, resulting in diseases that are difficult to manage, thus creating increased rates of pathogen/disease transmission, serious sickness, and mortality (Boral et al., 2019). In this review, we will focus our discussion only on AMR exhibited by bacteria. One of the prototype historical examples of resistance is Staphylococcus aureus. Over the decades, first in the 1940s, with the beginning of penicillinase production and penicillin resistance, followed by macrolides and tetracyclines in the 1950s, and finally methicillin in the early 1960s (Callaway et al., 2024). There are several genera and species of harmful bacteria classified as gram positive or gram negative. Bacteria that will be discussed in this review include Acinetobacter baumannii, an aerobic, non-motile, coccobacilli, gram-negative, and non-motile. Pseudomonas aeruginosa is an aerobic, oxidase positive, and gram-negative rod. Enterococcus faecalis is a gram-positive bacteria arranged in chains, facultative anaerobe, and a facultative pathogen. Staphylococcus aureus is a non-motile, gram-positive, facultative pathogen arranged in grape-like clusters. Some other examples of bacteria that are public health threats include Neisseria gonorrhea, Mycobacterium tuberculosis, Escherichia coli, and Clostridium difficile. Collecting dataThis narrative review was performed using Google Scholar, MEDLINE, NCBI, EBSCO, ScienceDirect, SCOPUS, and Web of Science databases. The areas of concern for AMR research were retrieved. The detailed literature on AMR in bacteria, its global prevalence, morbidity, and mortality, mechanisms, implications, and consequences were investigated. The criteria included in this article were, (i) English articles; (ii) published in the period of 1970 to February 2024. The exclusion criteria were as follows: (i) articles with inadequate data; (ii) outdated and obsolete data; (iii) review articles; and (iv) studies prior to 1970. Further information was obtained from the gray literature (books, YouTube videos, CDC reports, and books). Global prevalence of antibiotic resistanceAntibiotic resistance is more prevalent nowadays, and the consequent lack of efficient antimicrobials has become a global health concern (Velazquez-Meza et al., 2022). To address this concern, the World Health Organization (WHO) published its first list of antibiotic-resistant “priority pathogens” in 2017 with the aim of guiding and promoting research for new antibiotics (Lappin et al., 2017). The list categorizes about 12 families of bacteria into “critical,” “high”, and “medium.” P. aeruginosa, Enterococcus, S. aureus, A. baumannii, and E. coli belong to the critical group, as well as, they have become resistant to the most effective antibiotics used for multidrug-resistant bacteria. According to a review of the antimicrobial resistance (RAR) project, the fatality rate due to RAR was approximately 700,000 cases per annum (Townsend et al., 2020). WHO Global Action Plan on AMR (GAP-AMR) committee observed the frequency of antibiotic-resistant strains against ciprofloxacin. This antibiotic is frequently used to manage patients with gastrointestinal tract (GIT) and urinary tract infections (UTI) caused by some Enterobacteriaceae species. The resistance percentage exhibited by E. coli were approximately 9%–93% and that of Klebsiella pneumoniae were 4%–80% (Murray et al., 2022). Enterococci were observed to be one of the frequent etiologies isolated in healthcare-associated infections, and they account for 25%–50% of in-patient mortality rates. A meta-analysis study conducted to survey the frequency of antibiotic resistance showed that 125 studies out of 291 reported prevalence of E. faecalis antibiotic resistance, with quinupristin/dalfopristin having the highest rate (97%) (Bhattacharya et al., 2024). Morbidity and mortality due to AMRA recent high-profile report revealed that ten million people are likely to die from AMR by 2050 if the current situation is not controlled. However, due to the lack of comprehensive information on the incidence of AMR, morbidity, mortality, and modeling of future scenarios, this prediction was challenged (Knupp-Pereira et al., 2024). Drug-resistant pathogens like M. tuberculosis, which causes tuberculosis, have a serious negative impact on some Asian and African nations. Approximately 500,000 patients with multiple drug-resistant tuberculosis (MDR-TB) were identified in 2013 (Bhattacharya et al., 2024). Research also shows that Enterococcus faecium resistance to antibiotics such as linezolid is highest in Southeast Asia and lowest in America. However, AMR is a concern as well. In the US, the estimated morbidity of annually developed antibiotic-resistant infections is more than two million, leading to approximately 23,000 deaths. Methicillin resistant Staphylococcus aureus (MRSA) was noticed to be the predominant agent comprising 19,000 fatalities. According to surveillance data, Europe is comparable to the United States in terms of 25,000 antibiotic-related deaths per year (Knupp-Pereira et al., 2024). Bacteria are frequently emerging and acquire drug resistance; hence, this is extremely crucial to investigate the factors that favor the increase in AMR around the globe. AMR is aggravated because of self-medication and widespread antibiotic use. It is facilitated through easy availability over the counter, without prescriptions, and through unregulated supply chains (Orcid, 2019). Noncompliance with antibiotics either by mistake or deliberately, especially when symptoms subside, is one of the causes of AMR. Noncompliance exposes the surviving bacteria to subtherapeutic concentrations of antibiotics, increasing the likelihood of resistance development (Bhattacharya et al., 2024). Several factors contribute to morbidity and mortality due to AMR. This includes increased virulence, enhanced pathogenesis, and new mechanisms of antibiotic resistance like biofilm formation (Fig. 1). The threat of AMR was not confined to Asian and African nations; many Western countries were also concerned about the worldwide threat of AMR. The extensive use of prophylactic antibiotics prior to procedures in hospitals is one of the causes of this (Knupp-Pereira et al., 2024). Developed nations also have some similar factors as such as antibiotic misuse, irrelevant prescriptions by physicians, poor patient awareness, and patient lack of drug compliance. Inadequate hand hygiene as well as cross-transmission of infections between hospitalized patients have also been identified as causes of increased antibiotic resistance infections in hospitals. Sharing of antibiotics is also common, where an individual uses “leftover” antibiotics originally prescribed to another individual (Lee et al., 2023). Moreover, the excessive use of antibiotics in livestock, in for the treatment of infections, control of diseases in poultry, and enhancement of animal productivity has further increased the risk of AMR. Transmission of these bacteria occurs through consumption of the affected meat and colonization of the human gut with AMR microorganisms, inducing AMR in the already existing commensal GIT (Liang et al., 2020). AMR-resistant bacterial transmission might occur through contamination of feces/urine/animal products in drinking water as well as ingestion of contaminated foods.
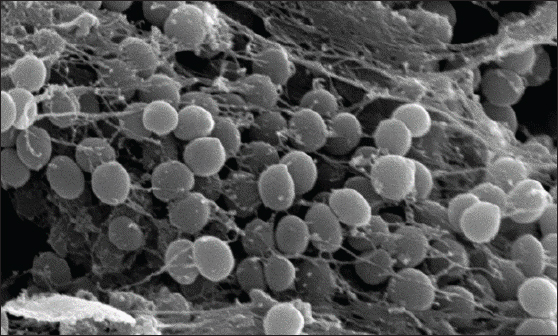
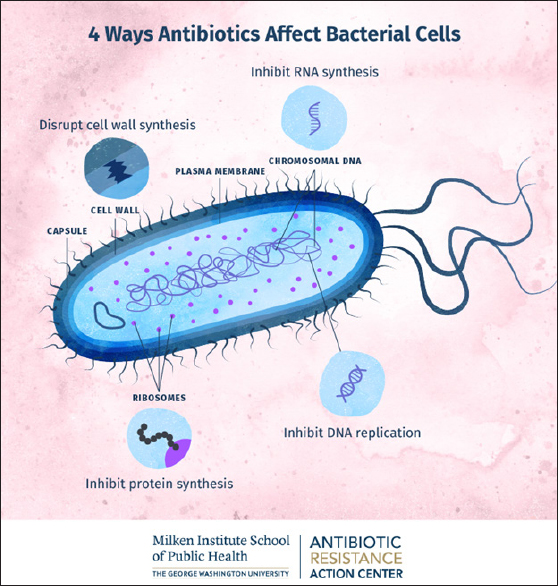
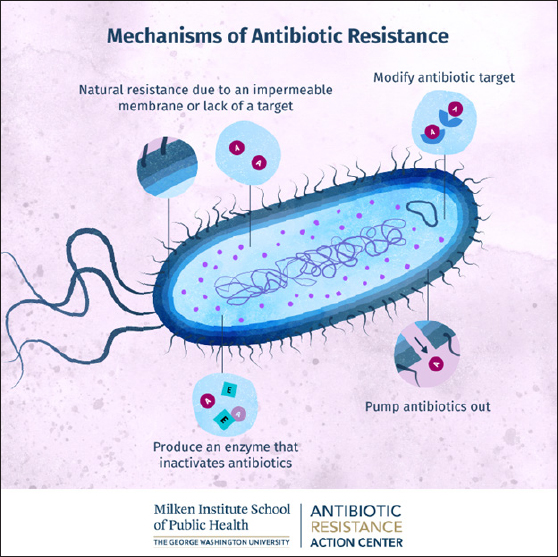
Fig. 1. Scanning electron micrograph of a bacterial biofilm (image courtesy: Meagan Walker, University of Guelph; Ameet Singh, University of Guelph, J. Scott Weese, Ontario Veterinary College, Guelph, Ontario, Canada). Mechanisms of antibiotic resistanceAntibiotics act on bacteria through four mechanisms. The mechanisms of antibiotic action include the inhibition of bacterial cell, plasma membrane, protein synthesis, and nucleic acids (Fig. 2). The origins of bacterial AMR can be divided into intrinsic and acquired/secondary resistance. In cases of intrinsic AMR, the genes are naturally occurring in the species and may always be expressed in the species or expressed only following exposure to an antibiotic (induced) (Alduina et al., 2020). Natural resistance mechanisms include decreased inflow of antibiotics through the plasma membrane, activation of active transporter/efflux pumps, and the nature of the bacterial cell wall. The activation of active transporters can also occur in induced resistance. Secondary/acquired AMR usually occurs through parallel gene transfer, where the acquisition of DNA/plasmid occurs through transformation, transposition, and conjugation (Murray et al., 2022). It can also occur through mutations in chromosomal DNA as a result of stressors such as starvation, ultraviolet radiation, and exposure to chemicals. The bacteria may acquire genetic material through plasmid-borne transmission, bacteriophage-borne transmission, or direct transmission from the external environment, such as in Actinobacter spp. Bacterial resistance can be measured using disc diffusion, E-test, and dilution tests. In dilution tests, the minimum amount of antibiotic that suppresses the growth of microorganisms can be measured. This is called the minimum inhibitory concentration (MIC), and the minimum amount of antibiotic that kills all bacteria (the minimum bactericidal concentration) can also be assessed. Bacteria may have intrinsic resistance if the mean MIC for the bacteria is under resistance or if the resistant genes are acquired from other microorganisms (Serretiello et al., 2023). The critical modes of AMR in bacteria include (i) decreased entry of antibiotics, (ii) modification of the binding site, (iii) inactivation of the antibiotic using enzymes, and (iv) efflux pumps/active transporters of the antibiotic (Fig. 3). Bacteria can naturally limit the uptake of antibiotics by cells. The cell wall (outer membrane) of Gram negative organisms provides the natural resistance for some antimicrobial agents (Torres Ortiz et al., 2021). One such example is mycobacteria, with its high lipid content outer membrane allowing limited uptake of hydrophilic drugs and hydrophobic drugs (for example, rifampicin and fluoroquinolones) having access through the wall. The cell wall-deficient bacteria Mycoplasma also possesses natural AMR to beta lactams as well as glycopeptides, both of which target the cell wall to produce its action (Woodford et al., 2011). Beta lactam drugs covalently bind to enzymes needed for the synthesis of bacterial cell walls, such as endopeptidases, transpeptidases, and carboxypeptidases. These enzymes are known as penicillin-binding protein (PBP). Resistance to -lactams through modification of the target site, namely PBPs, is a resistance mechanism applied by Gram-positive bacteria. Staphylococcus aureus, through the MecA gene, produces an altered PBP termed PBP2a, which has a lower affinity to beta lactams and, therefore, decreases or completely inhibits the binding of beta lactams (Iskandar et al., 2022).
Fig. 2. Mechanisms of antibiotic action on bacteria. (Image courtesy: Milken Institute School of Public Health). Several bacteria like S. aureus, P. aeruginosa, and E. coli may form polymeric particles that accumulate outside the bacterial cell. Within these aggregated substances, several bacterial species live. This is called a biofilm, which protects bacteria from the action of antimicrobial agents and other toxins (Fig. 1). The enhanced virulence exhibited by bacterial biofilms may result in severe clinical complications with fatal consequences (Coll et al., 2024). In many bacteria, biofilms may also result in the persistence of disease as well as resistance to prescribed antibiotics (Zhang et al., 2009). The recognized factors that allow organisms in biofilms to resist antibiotics include: (i) polymeric matrix restricting the entry of antimicrobials, (ii) preventing the contact of antimicrobials with biofilm, (iii) bacteria producing enzymes like penicillinase/extended spectrum beta lactamase (ESBL), (iv) bacteria inside the biofilm might alter its multiplication rate and respiration processes, (v) bacterial binding sites may get altered as well as these sites may not be exposed, (vi) removal of antimicrobials with the help of active transporters/efflux pumps, and (vii) possession of lipopolysaccharide (LPS) cell walls, generally prevents the action of beta lactam antibiotics. In addition, exopolysaccharide sheaths formed by biofilm-producing bacteria significantly decrease their antibiotic susceptibility. Such organisms include S. aureus, Enterococcus, and K. pneumoniae. The latter is considered to be a significant cause of health care-associated pneumonia associated with high morbidity and mortality (McCowan et al., 2022).
Fig. 3. Mechanisms of antibiotic resistance in bacteria. (Image courtesy: Milken Institute School of Public Health). Multidrug-resistant (MDR) bacteriaAcinetobacter baumannii Acinectobacter species are Gram-negative aerobic and ubiquitous bacteria (Aires et al., 1999). It is commonly found in soil and water and colonizes intravenous fluids (Liang et al., 2020). Despite the fact that there are more than 30 distinct species belonging to this genus, A. baumannii accounts for more than 90% of Acinetobacter isolates. Furthermore, A. baumannii has the greatest known clinical significance due to its increased involvement in hospital-acquired infections in intensive care units (ICUs) patients and its ability to develop pan-drug resistance (Lee et al., 2023). Acinetobacter baumannii predominantly infects critically ill patients and is known to cause multiple nosocomial infections, such as hospital-acquired pneumonia, central line-associated sepsis, osteomyelitis, necrotizing fasciitis, cellulitis, UTI after catheterization, and central nervous system (CNS) infections (Serretiello et al., 2023). These bacteria exhibit a remarkable course of action by accumulating numerous resistance elements within the bacterial cell (Iskandar et al., 2022). In addition, A. baumannii has an exceptional ability to survive in unfavorable conditions, increasing its susceptibility to spread as a nosocomial microorganism (Uddin et al., 2021). One of the most notorious virulence factors of A. baumannii is biofilm formation, which contributes to its endurance in harsh environments, such as hospitals and medical equipment. Biofilms are micro-communities of microorganisms surrounded by polymeric polysaccharides that exhibit antibiotic resistance, immunity evasion, and desiccation (Breijyeh et al., 2020). They also have a chaperon/ usher pili system controlled by the BfmRS two-component system, which influences gene expression and helps in the formation of a protective capsule in response to antibiotics (Serretiello et al., 2023). This has created various obstacles and epidemics in the treatment of diseases, especially in ICU patients. Although Acinectobacter species are nonmotile, A. baumannii strains are capable of locomotion by two methods: surface-associated motility and twitching motility. Another virulence factor contributing to its survival is the outer membrane protein ompA, which invades host cells and causes apoptosis. There is a bacterial protein with molecular weight 33-36 kDa called, (omp protein) that facilitates AMR against carbapenems. There are many other virulence factors possessed by A. baumannii (Iskandar et al., 2022). There are various mechanisms by which A. baumannii develops antibiotic resistance. One of the most common mechanisms of beta-lactam hydrolysis is via beta-lactamases. Ambler class A beta-lactamases can hydrolyze all penicillin and cephalosporins except cephamycins (Sartorius et al., 2024). Ambler class B penicillinases are known for hydrolyzing various types of and modified penicillin. In addition, A. baumannii inherently synthesizes another Ambler class penicillinase (C type/AmpC) that inactivates cephalosporin antibiotics. They are resistant to clavulanic acid but sensitive to ccephamycin (cefoxitin and ceftazidime). Ambler class beta lactamases are also known as oxacillinases and exhibit oxacillin and cloxacillin hydrolyzing activities. They can hydrolyze extended-spectrum cephalosporins and carbapenems (Coll et al., 2024). Tetracycline resistance is primarily caused by energy-dependent efflux pumps in A. baumannii. These bacteria possess two active transporters/efflux pumps that specifically flush out tetracyclines from the bacterial cell. They are called efflux pumps (TetA pumps) and efflux pumps (TetB/RND). TetA pumps exhibit AMR against tetracycline without conferring protection against doxycycline/minocycline. The second efflux pump (TetB) provides AMR against tetracycline as well as minocycline but not tigecycline (Uddin et al., 2021). DNA gyrase and DNA topoisomerase IV enzymes may undergo mutations in A. baumannii to acquire AMR to fluoroquinolones (Breijyeh et al., 2020). The development of aminoglycoside-modifying enzymes in A. baumannii leads to aminoglycoside resistance (AMEs). Such AMEs modify the aminoglycosides’ associated functional groups, making it harder for these antibiotics to bind to their ribosomal target sites. Currently, resistance to polymyxin E (colistin) in A. baumannii is increasing. Previously reported mechanisms of colistin resistance identified in this species include (i) alteration of the structure of the outer membrane’s lipid A by adding phosphoethanolamine, (ii) mutation of the genes synthesizing lipid A resulting in the absence of lipopolysaccharide, (iii) decreased exhibition of proteins needed for outer membrane protein (OMP) rigidity, and (iv) insufficient synthesis of cofactors for bacterial LPS (Ghimpețeanu et al., 2022). Pseudomonas aeruginosaThese bacteria are Gram-negative bacilli, motile with polar flagellum, oxidase positive, and heterotrophic in nature. It can spread via contaminated water and soil. In healthcare facilities, resistant strains of the pathogen can potentially transfer from one person to another via contaminated surfaces, tools, or hands. P. aeruginosa infection commonly occurs in hospitalized patients or those who are immunocompromised (Despotovic et al., 2023). P. aeruginosa can cause several types of infections. Localized infection with P. aeruginosa following burns can be identified due to the blue–green purulent discharge, and this infection may progress to cause sepsis. More than 60% of individuals with cystic fibrosis have a chronic infection with P. aeruginosa, which is associated with greater mortality (Ikhimiukor et al., 2022). Nerotizing P. aeruginosa pneumonia may develop following the use of contaminated respirators. Any patient with pneumonia symptoms and immunocompromised status should be evaluated for Pseudomonas pneumonia (Koch et al., 2021). In children, puncture wounds in the foot can cause pseudomonas infection, and delayed treatment can predispose them to osteomyelitis and septic arthritis. Furthermore, P. aeruginosa can cause corneal infection following eye surgery, and in patients with diabetes, it can cause malignant otitis externa (Zhou et al., 2022). Because of the ability of P. aeruginosa to withstand antibiotics, eradication has become more challenging (Lee et al., 2023). P. aeruginosa strains are well known for their elevated levels of intrinsic and acquired resistance mechanisms to fend off most antibiotics. Limiting membrane permeability to antimicrobials is a characteristic of intrinsic antibiotic resistance (Lee et al., 2023). Efflux mechanisms allow bacteria to transport toxic or damaging substances outside the cell membrane. Furthermore, many isolates have beta-lactamases and ESBLs. Another significant method by which Pseudomonas bacteria can develop resistance to antibiotics and evade host defenses is the formation of biofilms (Serretiello et al., 2023). The production of inactivating beta-lactamases is an imported mechanism of resistance to beta-lactams. The most common beta-lactamases are penicillinases (Mączyńska et al., 2023). However, these penicillinases have no effect on the clinical effectiveness of carbapenems, monobactam, or extended-spectrum cephalosporins. P. aeruginosa has also been found to include extended-spectrum-lactamases and carbapenem hydrolyzing enzymes. Furthermore, P. aeruginosa has developed resistance to aminoglycosides by inactivating the drug molecule enzymatically via chemical modification. These enzymes include aminoglycoside phosphoryl transferase, aminoglycoside acetyltransferase, and aminoglycoside nucleotidyl transferase. The enzymes phosphorylate, acetylate, and adenylate the drug molecules, respectively. Individual strains of P. aeruginosa have the potential to develop resistance to all aminoglycosides because they are able to carry the genes for several aminoglycoside-inactivating enzymes (Papadimitriou-Olivgeris et al., 2017). Enterococcus faecalisE. faecalis is a gram-positive cocci arranged in long chains, commensal, opportunistic, normally found in the GIT. It is commonly found in pairs and chains of diverse sizes. It is known to cause hospital-acquired infections, among which UTI are the most common (Callaway et al., 2024). It can also lead to endocarditis, wound infection, and severe septicemia. The pathogen normally affects persons with comorbidities or immunocompromised patients (Xu et al., 2021). Treatment of hospital-acquired E. faecalis infections is difficult because of frequent AMR to several antibiotics. Many cases of vancomycin-resistant Enterococci (VRE) have been reported. Because vancomycin is the ultimate treatment choice for most Gram-positive bacteria, it is an overly concerning development (Almaghrabi et al., 2024). The earliest reports of VRE cases were reported in the 1980s regarding GI tract diseases in animals and humans. E. faecalis is also known to be resistant to antibiotics such as ampicillin, linezolid, daptomycin, quinupristin-dalfopristin, and aminoglycosides (Murray et al., 2022). Hospital-acquired vancomycin-resistant E. faecalis is a great concern due to the great resilience of the pathogen. The patient can survive extended time in hospital environments such as operating theater, wards, and emergency rooms. These bacteria can withstand elevated temperatures and the use of disinfectants. These bacteria are naturally tolerant to many classes of antimicrobials. These bacteria readily accumulate genetic changes and genes from other bacteria. Enterococci evolved AMR by diverting antibiotics away from critical septal targets via cell membrane anionic phospholipid redistribution (Murray et al., 2022). Enterococcus species naturally exhibit AMR to many classes of antibiotics and can receive resistance genes (plasmids) from other bacteria and attain AMR to additional antibiotics (Bereket et al., 2012). Ampicillin-resistant enterococci, when exposed to ampicillin, produce PBP-5 that expresses less binding with this drug. Enterococcus naturally express less AMR against gentamicin/streptomycin (aminoglycosides) because these antibiotics enter the bacterial cell in lower amounts. However, resistant Enterococcus species acquire higher AMR by secreting enzymes that degrade these antibiotics (Murray et al., 2022). Enterococci resistant to vancomycin express low binding sites for this potent antibiotic, making this drug ineffective for bactericidal activity. Enterococci exhibiting AMR against quinupristin-streptogramin-dalfopristin (Q–D) drugs exhibit many mechanisms like, antibiotic degradation, less binding, altering the drug (by virginiamycin acetyl transferase (V-at), making the drug incapable through virginiamycin B-lysase (V-gb), and efflux pumps with macrolide-streptogramin resistant polypeptide (M-sr-C) (Coll et al., 2024). These enterococcal strains also possess resistance to oxazolidinone-linezolid through changes in 23S ribosomal-RNA affinity targets in the bacterial ribosome. Enterococci express AMR for daptomycin (protein with lipid-based drug) through changes in affinity and less permeability through glycerol-phosphoryl-diester-phospho-diesterase (Gd-p-D) cardiolipin-synthase (Cl-s) membrane-protein (Lia-F) (McHugh et al., 2022). Staphylococcus aureus (MRSA, vancomycin resistance S. aureus (VRSA))Staphylococcus aureus (S. aureus) is a commensal bacterium found in different areas like the nasal cavity, skin, GIT, vagina, and oral cavity (Kwon et al., 2024). S. aureus is catalase-positive, coagulase-positive, and gram-positive cocci arranged in vine-like clusters, and with a size 1 μm in diameter (Murray et al., 2022). Staphylococci belong to the family Staphylococcaceae, with the ability to cause a multitude of infections, the capacity to adapt to varying environmental conditions, and compelling etiological agents in healthcare-associated diseases (HCAD) as well as community-derived infections (Sartorius et al., 2024). This bacterium was the first to be described pathogen, and S. aureus infections are highly associated with blood infections, cellulitis, fasciitis, pneumonia, osteomyelitis, and UTI (Zhou et al., 2022). It may cause medical instrumentation-associated diseases like central-line–associated bloodstream infections, and severe invasive diseases like, meningitis (Murray et al., 2022). The bacteria are equipped with a number of pathogenic products like virulent enzymes (coagulase, catalase, DNase, and protease), enterotoxins, exfoliating toxins, toxic shock syndrome toxin-1, and so on (Pulingam et al., 2022). One major concern regarding this bacterium is the development of AMR toward many antibiotic categories. The first case of AMR staphylococci was observed with the first is S. aureus resistance emerging only 2 years after the initial prescription of beta-lactamase antibiotics. There is an increasing number of S. aureus resistance cases isolated from intensive care units and blood cultures to various antimicrobials (Parmanik et al., 2022). Methicillin was first introduced in the mid of 1900s, a semi-synthetic penicillinase-resistant beta lactam, used in treating penicillinase-forming S. aureus (Godijk et al., 2022). Within a short period, S. aureus strains resistant to this drug surfaced. MRSA is considered a prominent etiologic agent associated with HCAD. Compared with methicillin-susceptible-S. aureus, infections related to MRSA types are associated with extremely elevated death rates, morbidity, prolonged hospital admission, and increased economic loss. Initially, MRSA infections occur in patients with some form of hospital exposure (Kwon et al., 2024). However, an increasing number of infections have recently been observed in subjects without prior hospital exposure. Thus, MRSA infections were divided into healthcare-associated and community-acquired MRSA diseases. MRSA was noted to be resistant to beta-lactam antibiotics due to the mecA and mecC gene sequences located on the chromosomal cassette, which produces altered PBP, termed PBP2a and PBP2aLGA. Compared with native PBPs, these altered PBPs produce low amounts of penicillinase acylation and less binding to the penicillin group of antibiotics (Murray et al., 2022). MRSA is susceptible to vancomycin, a tricyclic glycopeptide antibiotic, and hence, it is a last-resort therapeutic agent for these strains. However, because of the increased use of vancomycin against MRSA strains, VRSA emerged (Sartorius et al., 2024). The drug exerts its bactericidal effects on enzymes involved in the formation of the bacterial cell wall in MRSA and other bacteria. This mechanism occurs by altering H2 bond association in the last Dalanyl-Dalanine (dAla–dAla) structures in the active lipid, preventing the initial molecules from binding to the growing mucopeptide, culminating in degradation of the bacterial cell wall and cell death. The gene responsible for vancomycin resistance is the van gene, which is divided into 11 gene clusters (VanF, VanE, VanD, VanL, VanI, VanG, VanM, VanC, VanB, VanN, and VanA). In S. aureus, the VanA genetic group was associated with vancomycin-resistant species (Murray et al., 2022). This gene cluster codes for five proteins (VanX, VanR, VanH, VanA, and VanS), which produce the resistance patterns in VRSA. VanX, VanA, and VanH modify the initial molecule, Dalanine-Dalanine (DAla-Dala), of the peptidoglycan cell wall into D-Ala-D-Lac. D-Ala-D-Lac changes D-Ala-D-Ala in the formation of the peptidoglycan cell wall, which culminates in a thousand-fold dip in the binding with vancomycin to form hydrogen bonds, thereby resulting in the loss of the bactericidal effect produced by vancomycin (Bereket et al., 2012). Neisseria gonorrhea (Gonococci)Gonococci are oxidase-positive cocci arranged in pairs, intracellular, and obligate aerobes. The mode of transmission is usually spread through sexual contact between individuals. Because an organism cannot survive well outside its host, direct, intimate contact is necessary for transmission (Demissie et al., 2024). It is also known to cause disease when a pregnant woman is infected with a baby during birth and can lead to gonococci ophthalmia neonatorum. Gonorrhea is a predominant agent of sexually transmitted infections (STIs) and is observed worldwide. Infection with these bacteria leads to gonorrhea, a disease in both males and females (Edwards et al., 2000). Most patients infected with N. gonorrhea remain asymptomatic and transmit the infection to others. Gonorrhea can lead to local symptoms such as urethral and vaginal discharge and dysuria (Quillin and Seifert, 2018). Untreated, gonorrhea can cause disseminated disease and complications, particularly in women. It includes stricture in the fallopian tube, leading to infertility, ectopic pregnancy, pelvic inflammatory disease, and so on (Ginindza et al., 2017). Diseases caused by N. gonorrhea, especially can lead to high fatality rates. The high prevalence associated with this bacterium is noticed as highest in individuals of reproductive age who are sexually active. The rates of infertility and risk of co-infection are high among individuals who acquire the infection. Antibiotic resistance in N. gonorrhea has been on the rise since the 1930s, when the first isolates resistant to sulfonamides were discovered. N. gonorrhea is known to be resistant to Penicillin, tetracyclines, macrolides, spectinomycin, fluoroquinolones, azithromycin, and third-generation cephalosporins (Unemo et al., 2019). Recently, cases of ceftriaxone-resistant isolates were detected in Belgium and Portugal. Gonococci possess the ability to change DNA through mutation, particularly by coding for its pili protein. The pathogen develops AMR through gene transfer through conjugation, transduction, and bacteriophage transformation (Francis et al., 2018). Through these means of mutations, N. gonorrhea has developed all the known psychological mechanisms for AMR to all the antimicrobials mentioned above, which are used for treatment. A pathogen can 1) destroy or modify an antibiotic by enzymatic actions, 2) modify or reduce the affinity of the target site of an antibiotic, 3) decrease the influx of antimicrobials, and 4) increase the efflux of antimicrobials (Murray et al., 2022). Mycobacterium tuberculosis (MDR and XDR)Mycobacterium tuberculosis is an acid-fast bacterium (AFB), slow-growing, Niacin-positive, and mycolic acid-positive bacterium that contains mycolic acid in its cell wall. This bacterium belongs to the family Mycobacteriaceae and is associated with multiple presentations of TB in humans (TB) (Dorji et al., 2024). Mycobacterium tuberculosis lacks the characteristic features of gram-positive/gram-negative cell walls; however, the presence of mycolic acid in mycobacteria, provides unique features like the AFB staining property, slow growth in vivo-in vitro and is associated with virulence. Mycolic acid/waxy substances in the cell wall prevent phagocytosis by alveolar macrophages and other phagocytes in the body (Torres Ortiz et al., 2021). In Asia, Africa, and Latin America, most people are already infected by M. tuberculosis; however, they present as latency. Due to their normal immune status, the disease may not progress further but, this disease TB poses a serious threat to humankind in modern times (Liu et al. 2024). M. tuberculosis organisms exhibit AMR to anti-TB medications used at the first stage and cause drug-resistant tuberculosis. Multiple antibiotic/drug-resistant TB (MDR) are mycobacteria that express resistance to minimum anti-tuberculosis drugs, rifampicin, and isoniazid (Omar et al., 2024). M. tuberculosis strains are resistant to all available anti-TB drugs, including first- and second-level drugs. These strains of M. tuberculosis are known as extremely drug-resistant TB (XDR-TB). XDR-TB is also resistant to fluoroquinolone as well as TB drugs administered by injection. Nearly 500,000 patients with TB were tested positive for sputum in developing countries, of which 10% were classified as XDR-TB (Hazra et al., 2023). Drug-resistant mycobacteria (M. tuberculosis), both MCRTB and XDRTB, create biothreat-like situations to prevent the containment of TB outbreaks globally. In 2018, the World Health Organization announced that 500,000 new cases of TB caused by rifampicin resistance were noticed (Singha et al., 2024). Since then, there has been a surge in MDR, and XDR-TB cases were reported in developing as well as developed countries. XDR-TB cases were reported from the USA, Germany, the UK, Australia, Canada, and several other Western countries. In 2019, 20%–35% of MDR-TB cases were reported from Western countries. The high incidence of MDR-TB and XDR-TB was attributed to immunosuppression (AIDS), chemotherapy, radiotherapy, organ transplantation, and other factors like Diabetes mellitus. In developing countries, the increased rate of drug-resistant tuberculosis was due to AIDS, malnutrition, famine, and drought (Hashemzadeh et al., 2024). M. tuberculosis acquires MDR and Extremely drug resistant (XDR) through transformation, a mutation in the nucleoid, and transduction using mycophages, and plasmids. The AMR TB strains can alter the conventional and new TB drugs by secreting enzymes, changing the binding site, and causing drug modification, and destruction. Many MDR and XDR TB strains possess effective drug-flushing mechanisms using efflux pumps (Chizimu et al., 2023). Escherichia coli (E. coli)E. coli strains are motile, contain peritrichous flagella, bacilli, are Gram-negative, and are oxidase negative and belong to the family Enterobacteriaceae. This bacterium is commensal and is present predominantly in human and animal GIT and excreted in feces in large numbers (Pan et al., 2024). E. coli can cause a variety of diseases, such as UTI, gastroenteritis, neonatal meningitis, hemolytic uremic syndrome (HUS), meningitis, pneumonia, liver abscess, and other diseases (McCowan et al., 2022). E. coli can be transmitted as water-borne as well as food-borne disease. There are six types of E. coli identified in gastroenteritis: (i) enteropathogenic E. coli (EPEC), (ii) enterohemorrhagic E. coli (EHEC), (iii) enteroinvasive E. coli (EIEC), (iv) enterotoxigenic E. coli (ETEC), (v) enteroaggregative E. coli (EAgEC), and (vi) Diffusely adhesive E. coli (DAEC). Diarrhea is caused by ETEC strains in persons from Western countries visiting. The complication of EHEC is HUS, which is a profoundly serious condition with irreversible kidney damage and renal failure (Gomi et al., 2024). Multi-drug-resistant E. coli cases are frequently reported from many parts of the globe very frequently (Jin et al., 2024). These strains are resistant to ciprofloxacin, amoxicillin, tetracycline, aminoglycosides, co-trimoxazole, fusidic acid, nalidixic acid, amoxicillin-clavulanate, and cefuroxime (Pereira et al., 2024). These AMR-producing E. coli are known to produce penicillinase as well as ESBL. ESBL-E. coli strains can form a single beta lactamase that hydrolyzes many potent classes of beta lactam antibiotics (Murray et al., 2022). Many AMR strains of E. coli are also resistant to third- and fourth-generation cephalosporins. These resistant strains are highly associated with healthcare infections/nosocomial diseases (Pan et al., 2024). Clostridioides difficileClostridium difficile is a ubiquitous, anaerobe, spore-producing, nonmotile, and gram-positive bacterium (Abdrabou et al., 2022). This bacterium was previously termed C. difficile, which is a normal commensal bacterium in human and animal intestines. This bacterium is highly known for causing pseudomembranous colitis, usually after a history of antibiotics (antibiotic-associated diarrhea) (Oliveira et al., 2024). The virulent C. difficile strains secrete enterotoxins responsible for gastroenteritis. This bacteria inherently produces spores that are resistant to heat as well as antibiotics (Liu et al. 2023). These bacteria (10.5%) were frequently identified in nosocomial environments, in ICU, patient bedside, nursing stations, and burn wards (Guh et al., 2020). C. difficile strains isolated from various parts of the world exhibited resistance to commonly used antibiotics such as cephalosporins, penicillin, tetracycline, co-trimoxazole, fusidic acid, nalidixic acid, amoxicillin, and amoxicillin-clavulanate, as well as many third-generation and some fourth-generation cephalosporins (Mastrantonio and Rupnik, 2024). In 2017, more than 223,900 patients were hospitalized due to AMR C. difficile strains, of which 12,800 were reported to have died (Vendrik et al., 2022). These strains can alter the drug, modify the binding site, secrete enzymes, and prevent the entry of antibiotics into bacterial cells. These bacteria can also acquire genes from related bacteria through conjugation, transformation, and transduction (mediated through bacteriophages) (Zbylicki et al., 2024). Implications and consequences of AMRThe therapeutic efficacy of antibiotics against pathogens is limited because of antibiotic resistance (Spigaglia et al., 2024). Individuals, healthcare institutions, and society will be affected by the consequences of AMR. Patients with AMR infections experience serious illness, which prolongs and complicates their hospital stay and, in turn, increases the admission costs and the need for newer second-line drugs, which again increases the cost (Tsai et al., 2023). This increasing burden of caring for suffering will increase the trouble for families, the community, and the healthcare systems (Seekatz et al., 2022). According to the CDC, approximately 2,000,000 clinical cases due to AMR strains are encountered around the world every year (Knight et al., 2021). The WHO and CDC noted the significance of AMR in bacteria associated with extremely high mortality and morbidity (Alam et al., 2023). It is also difficult to estimate the cost of public health expenditure and the impact associated with AMR in terms of mortality. Furthermore, there is a shortage of effective drugs available to treat multidrug resistance, and newer antibiotics need to be developed to limit AMR (Medrano et al., 2024). AMR is a serious concern and a severe public health bioheat worldwide (Maddock et al., 2024). Many parameters are associated with AMR in bacteria. A collaborative effort should be made between healthcare staff, public health officials, and policy makers (Velazquez-Meza et al., 2022). Preventive measures should be taken in as well as to curb the spread of AMR (Coll et al., 2024). Otherwise, there may be an outbreak of AMR strains of bacteria for which antibiotics would not be available for treatment. The efforts should include all healthcare levels up to patient awareness and community education (Knupp-Pereira et al., 2024). International agencies like the UN, WHO, and CDC, governments in all countries, regional governments, and local bodies/municipals should take appropriate steps and protocols to address the issue of AMR. Otherwise, this bioheat caused by AMR may culminate in irreversible health-associated catastrophes around the globe. ConclusionAMR in bacteria is increasing worldwide at an alarming rate. Millions of cases of AMR bacteria are reported from various parts of the world. These bacteria rare resistant to many antibiotics and are difficult to contain, treat, and manage. These strains pose extremely high mortality and morbidity in developing as well as developed countries. All international agencies, national governments, and local health authorities should develop guidelines and strategies to prevent further aggravation of AMR. AMR in bacteria, in addition to increased fatality, can affect a country’s economy and may result in a financial crisis. AcknowledgementsThe authors acknowledge the staff of the School of Medicine, Maldives National University for their support in the preparation of this article. Conflict of interestThe authors declare no conflict of interest. FundingNone. ReferencesAbdrabou, A.M.M., Bischoff, M., Mellmann, A., von Müller, L., Margardt, L., Gärtner, B.C., Berger, F.K. and German C. difficile surveillance group. 2022. Implementation of a Clostridioides difficile sentinel surveillance system in Germany: first insights for 2019–2021. Anaerobe 77, 102548; doi:10.1016/J.ANAEROBE.2022.102548. Aires, J.R., Köhler, T., Nikaido, H. and Plésiat, P. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43(11), 2624–2628; doi:10.1128/aac.43.11.2624 Alam, M., Saleem, Z., Haseeb, A., Qamar, M.U., Sheikh, A., Almarzoky Abuhussain, S.S., Iqbal, M.S., Raees, F., Chigome, A., Cook, A., Moore, C.E., Mustafa, Z.U., Salman, M., Saleh, U., Shabbir, S. and Godman, B. 2023. Tackling antimicrobial resistance in primary care facilities across Pakistan: current challenges and implications for the future. J. Infect. Public Health 16, 97–110; doi:10.1016/j.jiph.2023.10.046 Alduina, R., Gambino, D., Presentato, A., Gentile, A., Sucato, A., Savoca, D., Filippello, S., Visconti, G., Caracappa, G., Vicari, D. and Arculeo, M. 2020. Is caretta caretta a carrier of antibiotic resistance in the mediterranean sea? Antibiotics 9(3), 116; doi:10.3390/antibiotics9030116 Almaghrabi, R.S., Macori, G., Sheridan, F., McCarthy, S.C., Floss-Jones, A., Fanning, S., Althawadi, S., Mutabagani, M., Binsaslloum, A., Alrasheed, M., Almohaizeie, A., Allehyani, B., Alghofaili, A., Bohol, M.F. and Al-Qahtani, A.A. 2024. Whol-genomee sequencing of resistance and virulence genes in multidrug resistant Pseudomonas aeruginosa. J. Infect. Public Health 17(2), 299–307; doi:10.1016/J.JIPH.2023.12.012 Bereket, W., Hemalatha, K., Getenet, B., Wondwossen, T., Ali, S., Zeynudin, A. and Kannan, S. 2012. Update on bacterial nosocomial infections. Eur. Rev. Med. Pharmacol. Sci. 16(8), 1039–1044. Available via https://europepmc.org/article/med/22913154 Bhattacharya, R., Bose, D., Gulia, K. and Jaiswal, A. 2024. Impact of AMR on sustainable development goals and integrated strategies for meeting environmental and socio-economic targets. Environ. Prog. Sustain. 43(1), e14320; doi: 10.1002/EP.14320 Boral, B., Unaldi, Ö., Ergin, A., Durmaz, R., Eser, Ö.K. and Acinetobacter Study Group. 2019. A prospective multicenter study on the evaluation of antimicrobial resistance and molecular epidemiology of multidrug-resistant Acinetobacter baumannii infections in intensive care units with clinical and environmental features. Ann. Clin. Microbiol. Antimicrob. 18(1), 1–9; doi:10.1186/S12941-019-0319-8/FIGURES/3 Breijyeh, Z., Jubeh, B. and Karaman, R. 2020. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 25(6), 1340; doi:10.3390/MOLECULES25061340 Callaway, R., Johura, F.-T., Sultana, M., Sadique, A., Monira, S., Sack, D.A., Sack, R.B., Alam, M. and Chakraborty, S. 2024. Antimicrobial resistance of enterotoxigenic Escherichia coli from diarrheal patients and the environment in two geographically distinct rural areas in Bangladesh over the years. Microorganisms 12(2), 301; doi:10.3390/MICROORGANISMS12020301 Chizimu, J.Y., Solo, E.S., Bwalya, P., Kapalamula, T.F., Mwale, K.K., Squarre, D., Shawa, M., Lungu, P., Barnes, D.A., Yamba, K. and Mufune, T. 2023. Genomic analysis of Mycobacterium tuberculosis strains resistant to second-line anti-tuberculosis drugs in Lusaka, Zambia. Antibiotics 12(7), 1126; doi:10.3390/ANTIBIOTICS12071126/S1 Coll, F., Gouliouris, T., Blane, B., Yeats, C.A., Raven, K.E., Ludden, C., Khokhar, F.A., Wilson, H.J., Roberts, L.W., Harrison, E.M., Horner, C.S., Le, T.H., Nguyen, T.H., Nguyen, V.T., Brown, N.M., Holmes, M.A., Parkhill, J., Estee Török, M and Peacock, S.J. 2024. Antibiotic resistance using Enterococcus faecium whole-genome sequences: a diagnostic accuracy study using genotypic and phenotypic data. Lancet Microb 5(2), e151–e163; doi:10.1016/S2666-5247(23)00297-5 Demissie, E., Amare, A., Birhanu, M. and Gizachew, M. 2024. Neisseria gonorrhea antimicrobial resistance patterns and associated risk factors in women of childbearing potential in northwestern Ethiopia. BMC Women’s Health 24(1), 1–11; doi:10.1186/S12905-024-02898-3/TABLES/5 Despotovic, M., de Nies, L., Busi, S.B. and Wilmes, P. 2023. Reservoirs of antimicrobial resistance in the context of One Health. Curr. Opin. Microbiol. 73; doi:10.1016/J.MIB.2023.102291 Dorji, T., Horan, K., Sherry, N.L., Tay, E.L., Globan, M., Viberg, L., Bond, K., Denholm, J.T., Howden, B.P. and Andersson, P. 2024. Whole-genome sequencing of drug-resistant Mycobacterium tuberculosis isolates from Victoria, Australia. Int. J. Infect. Dis. 138, 46–53; doi:10.1016/J.IJID.2023.11.010 Edwards, J.L., Shao, J.Q., Ault, K.A. and Apicella, M.A. 2000. Neisseria gonorrhea elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect Immun. 68(9), 5354–5363; doi:10.1128/iai.68.9.5354-5363.2000 Francis, S.C., Mthiyane, T.N., Baisley, K., Mchunu, S.L., Ferguson, J.B., Smit, T., Crucitti, T., Gareta, D., Dlamini, S., Mutevedzi, T., Seeley, J., Pillay, D., McGrath, N. and Shahmanesh, M. 2018. Prevalence of sexually transmitted infections among young people in South Africa: a nested survey of a health and demographic surveillance site. PLoS Med. 15(2), e1002512; doi:10.1371/journal.pmed.1002512 Ghimpețeanu, O.M., Pogurschi, E.N., Popa, D.C., Dragomir, N., Drăgotoiu, T., Mihai, O.D. and Petcu, C.D. 2022. Antibiotic use in livestock and residues in food—a public health threat: a review. Foods 11(10), 1430; doi:10.3390/FOODS11101430 Ginindza, T.G., Stefan, C.D., Tsoka-Gwegweni, J.M., Dlamini, X., Jolly, P.E., Weiderpass, E., Broutet, N. and Sartorius, B. 2017. Prevalence and risk factors of sexually transmitted infections (STIs) among women of reproductive age in Swaziland. Infect. Agents Cancer. 12(1), 1–12; doi:10.1186/s13027-017-0140-y Godijk, N.G., Bootsma, M.C.J. and Bonten, M.J.M. 2022. Transmission routes of antibiotic resistant bacteria: a systematic review. BMC Infect. Dis. 22(1), 482; doi: 10.1186/S12879-022-07360-Z Gomi, R., Matsumura, Y., Yamamoto, M., Tanaka, M., Komakech, A.J., Matsuda, T. and Harada, H. 2024. Genomic surveillance of antimicrobial-resistant Escherichia coli in fecal sludge and sewage in Uganda. Water Res. 248, 120830; doi:10.1016/J.WATRES.2023.120830 Guh, A.Y., Mu, Y., Winston, L.G., Johnston, H., Olson, D., Farley, M.M., Wilson, L.E., Holzbauer, S.M., Phipps, E.C., Dumyati, G.K., Beldavs, Z.G., Kainer, M.A., Karlsson, M., Gerding, D.N. and McDonald, L.C. 2020. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N. Engl. J. Med. 382(14), 1320–1330; doi:10.1056/NEJMOA1910215 Hashemzadeh, M., Dezfuli, A.A.Z., Khosravi, A.D., Khosravi, N.A. and Saki, M. 2024. Antibiotic resistance and genomic characterization of Mycobacterium abscessus complex isolates from patients with pulmonary tuberculosis in Iran: a multicenter study (2010–2021). Acta. Microbiol. Imm. H. 1(aop), 82–88; doi:10.1556/030.2024.02169 Hazra, D., Lam, C., Chawla, K., Sintchenko, V., Dhyani, V.S. and Venkatesh, B.T. 2023. Impact of whole-genome sequencing of Mycobacterium tuberculosis on treatment outcomes for MDR-TB/XDR-TB: a systematic review. Pharmaceutics 15(12), 2782; doi:10.3390/PHARMACEUTICS15122782/S1 Ikhimiukor, O.O., Odih, E.E., Donado-Godoy, P. and Okeke, I.N. 2022. A bottom-up view of antimicrobial resistance transmission in . Nat. Microbiol. 7(6), 757–765; doi:10.1038/S41564-022-01124-W Iskandar, K., Murugaiyan, J., Hammoudi Halat, D., Hage, S.E., Chibabhai, V., Adukkadukkam, S., Roques, C., Molinier, L., Salameh, P. and Van Dongen, M. 2022. Antibiotic discovery and resistance: the chase and the race. Antibiotics 11(2), 182; doi:10.3390/ANTIBIOTICS11020182 Jin, C., Jia, C., Hu, W., Xu, H., Shen, Y. and Yue, M. 2024. Predicting antimicrobial resistance in E. coli using discriminative position-fused deep learning classifier. Comput. Struct. Biotechnol. J. 23, 559–565; doi:10.1016/J.CSBJ.2023.12.041 Knight, G.M., Glover, R.E., McQuaid, C.F., Olaru, I.D., Gallandat, K., Leclerc, Q.J., Fuller, N.M., Willcocks, S.J., Hasan, R., van Kleef, E. and Chandler, C.I. 2021. Antimicrobial resistance and COVID-19: Intersections and implications. Elife 10, e64139. Knupp-Pereira, P.A., Cabral, A.S., Dolores, Í.M., da Silva, A.B., Póvoa, H.C.C. and Neves, F.P.G. 2024. Antimicrobial resistance in Streptococcus pneumoniae before and after the introduction of pneumococcal conjugate vaccines in Brazil: a systematic review. Antibiotics 13(1), 66; doi:10.3390/ANTIBIOTICS13010066/S1 Koch, N., Islam, N.F., Sonowal, S., Prasad, R. and Sarma, H. 2021. Environmental antibiotics and resistance genes as emerging contaminants: methods of detection and bioremediation. Curr. Res. Microb. Sci. 2(11), 100027; doi:10.1016/j.crmicr.2021.100027 Kwon, J., Pelletiers, W., Galloway Peña, J., van Duin, D., Ledbetter, L., Baum, K., Ruffin, F., Knisely, J.M., Bizzell, E., Fowler, V.G., Chambers, H.F., Pettigrew, M.M. and Group for the A. R. L. (2024). Participant diversity in United States randomized controlled trials of antibacterials for Staphylococcus aureus infections, 2000–2021. Clin. Infect. Dis. 79(1), 141–147; doi:10.1093/CID/CIAE049 Lappin, M.R., Blondeau, J., Boothe, D., Breitschwerdt, E.B., Guardabassi, L., Lloyd, D.H., Papich, M.G., Rankin, S.C., Sykes, J.E., Turnidge, J. and Weese, J.S. 2017. Antimicrobial use guidelines for the treatment of respiratory tract disease in dogs and cats: antimicrobial guidelines working group of the International Society for Companion Animal Infectious Diseases. J. Vet. Intern. Med. 31(2), 279–294; doi:10.1111/JVIM.14627 Lee, J.H., Kim, N.H., Jang, K.M., Jin, H., Shin, K., Jeong, B. C., Kim, D.W. and Lee, S.H. 2023. Prioritization of critical factors for surveillance of the dissemination of antibiotic resistance in Pseudomonas aeruginosa: a systematic review. Int. J. Mol. Sci. 24(20), 15209; doi:10.3390/IJMS242015209/S1 Liang, Z., Yu, Y., Ye, Z., Li, G., Wang, W. and An, T. 2020. Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens in Pearl River Estuary and their exposure risk to human beings. Environ. Int. 143, 105934; doi:10.1016/j.envint.2020.105934 Liu, C., Monaghan, T., Yadegar, A., Louie, T. and Kao, D. 2023. Insights into the epidemiology of Clostridioides difficile infection and treatment: a global perspective. Antibiotics 12(7), 1141; doi:10.3390/ANTIBIOTICS12071141 Liu, B., Su, P., Hu, P., Yan, M., Li, W., Yi, S., Chen, Z., Zhang, X., Guo, J., Wan, X., Wang, J., Gong, D., Bai, H., Wan, K., Liu, H., Li, G. and Tan, Y. 2024. Prevalence, transmission, and genetic diversity of pyrazinamide resistance among multidrug-resistant Mycobacterium tuberculosis Isolates in Hunan, China. Infect. Drug Resist. 17, 403–416; doi:10.2147/IDR.S436161 Mączyńska, B., Jama-Kmiecik, A., Sarowska, J., Woronowicz, K., Choroszy-Król, I., Piątek, D. and Frej-Mądrzak, M. 2023. Changes in the antibiotic resistance of Acinetobacter baumannii and Pseudomonas aeruginosa clinical isolates in a multi-profile hospital in years 2017–2022 in Wroclaw, Poland. J. Clin. Med. 12(15), 5020; doi:10.3390/JCM12155020 Maddock, K.J., Burbick, C.R., Cole, S.D., Daniels, J.B., LeCuyer, T.E., Li, X.-Z., Loy, J.D., Sanchez, S., Stenger, B.L.S. and Diaz-Campos, D. 2024. A One Health perspective on the use of genotypic methods for antimicrobial resistance prediction. J. Am. Vet. Med. Assoc. 262(3), 303–312; doi:10.2460/JAVMA.23.12.0687 Mastrantonio, P. and Rupnik, M. (eds.). 2024. Updates on Clostridioides difficile in Europe, vol. 1435. Cham, Switzerland: Springer; doi:10.1007/978-3-031-42108-2 McCowan, C., Bakhshi, A., McConnachie, A., Malcolm, W., Sje, B., Santiago, V.H. and Leanord, A. 2022. E. coli bacteremia and antimicrobial resistance following antimicrobial prescription for urinary tract infection in: the community. BMC Infect. Dis. 22(1), 1–10; doi10.1186/S12879-022-07768-7/TABLES/7 McHugh, M.P., Parcell, B.J., Pettigrew, K.A., Toner, G., Khatamzas, E., El Sakka, N., Karcher, A.M., Walker, J., Weir, R., Meunier, D., Hopkins, KL., Woodford, N., Templeton, K.E., Gillespie, S.H. and Holden, M.T.G. 2022. Presence of optrA-mediated linezolid resistance in multiple E. faecalis lineages and plasmids revealed by long read sequencing. Microbiology (United Kingdom) 168(2), 001137; doi:10.1099/MIC.0.001137 Medrano, H., Lee, L., Young, V., Janecko, N., Deckert, A.E., Gow, S.P., Reid-Smith, R.J. and Agunos, A. 2024. Surveillance of antimicrobial resistance in Escherichia coli, Salmonella, and Campylobacter recovered from laying hens, their environment, and products in Canada indicated a stable level of resistance to critically important antimicrobials over varying time periods between 2007 and 2021. Int. J. Food Microbiol. 412, 110541; doi:10.1016/J.IJFOODMICRO.2023.110541 Murray, C.J., Ikuta, K.S., Sharara, F., Swetschinski, L., Robles Aguilar, G., Gray, A., Han, C., Bisignano, C., Rao, P., Wool, E., Johnson, S.C., Browne, A.J., Chipeta, M.G., Fell, F., Hackett, S., Haines-Woodhouse, G., Kashef Hamadani, B.H., Kumaran, E.A.P., McManigal, B., … Naghavi, M. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet, 399(10325), 629–655; doi:10.1016/S0140-6736(21)02724-0. Erratum in: Lancet, 400(10358):1102. doi: 10.1016/S0140-6736(21)02653-2. Oliveira, R., Tavares de Sousa, H. and Roseira, J. 2024. P1239 characterization of Clostridioides strains and antibiotic resistance profile in Inflammatory Bowel Disease patients with Clostridioides infection. J Crohns Colitis 18(Supplement_1), i2192–i2192; doi:10.1093/ECCO-JCC/JJAD212.1369 Omar, S.V., Louw, G., Ismail, F., Liu, X., Ngcamu, D., Gwala, T., van der Meulen, M. and Joseph, L. (2024). Performance evaluation of the Xpert MTB/XDR test for the detection of drug resistance to Mycobacterium tuberculosis among people diagnosed with tuberculosis in South Africa. MedRxiv 2024.02.16.24302824; doi:10.1101/2024.02.16.24302824 Orcid, R. 2019. Tackling antimicrobial resistance 2019-2024 – The UK’s five-year national action plan. J. Hosp. Infect. 101(4), 426–427; doi:10.1016/j.jhin.2019.02.019 Pan, F., Altenried, S., Scheibler, S. and Ren, Q. 2024. A rapid and specific antimicrobial resistance detection of Escherichia coli via magnetic nanoclusters. Nanoscale 16(6), 3011–3023; doi:10.1039/D3NR05463B Papadimitriou-Olivgeris, M., Fligou, F., Spiliopoulou, A., Koutsileou, K., Kolonitsiou, F., Spyropoulou, A., Zotou, A., Marangos, M., Anastassiou, E.D., Christofidou, M. and Spiliopoulou, I. 2017. Risk factors and predictors of carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii mortality in critically ill bacteraemic patients over a 6-year period (2010–15): antibiotics do matter. J. Med. Microbiol. 66(8), 1092–1101; doi:10.1099/jmm.0.000538 Parmanik, A., Das, S., Kar, B., Bose, A., Dwivedi, G.R. and Pandey, M.M. 2022. Current treatment strategies against multidrug-resistant bacteria: a review. Curr. Microbiol. 79(12), 388; doi:10.1007/s00284-022-03061-7 Pereira, A., Sidjabat, H.E., Davis, S., Vong da Silva, P.G., Alves, A., Dos Santos, C., Jong, J.B.D.C., da Conceição, F., Felipe, N.J., Ximenes, A., Nunes, J., Fária, I.D.R., Lopes, I., Barnes, T.S., McKenzie, J., Oakley, T., Francis, J.R., Yan, J. and Ting, S. 2024. Prevalence of antimicrobial resistance in Escherichia coli and Salmonella species isolates from chickens in live bird markets and boot swabs from layer farms in Timor-Leste. Antibiotics 13(2), 120; doi:10.3390/ANTIBIOTICS13020120 Pulingam, T., Parumasivam, T., Gazzali, A.M., Sulaiman, A.M., Chee, J.Y., Lakshmanan, M., Chin, C.F. and Sudesh, K. 2022. Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance, and strategies to overcome. Eur. J. Pharm. Sci. 170(1), 106103; doi:10.1016/J.EJPS.2021.106103 Quillin, S.J. and Seifert, H.S. 2018. Neisseria gonorrhea host adaptation and pathogenesis. Nat. Rev. Microbiol. 16(4), 226–240; doi:10.1038/nrmicro.2017.169 Sartorius, B., Gray, A.P., Davis Weaver, N., Robles Aguilar, G., Swetschinski, L.R., Ikuta, K.S., Mestrovic, T., Chung, E., Wool, E.E., Han, C., Gershberg Hayoon, A., Araki, D.T., Abd-Elsalam, S., Aboagye, R.G., Adamu, L.H., Adepoju, A.V., Ahmed, A., Akalu, G.T., Akande-Sholabi, W., … Naghavi, M. (2024). The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. Lancet Glob. Health 12(2), e201–e216; doi:10.1016/S2214-109X(23)00539-9 Seekatz, A.M., Safdar, N. and Khanna, S. 2022. The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection. Therap. Adv. Gastroenterol. 15, 17562848221134396; doi:10.1177/17562848221134396 Serretiello, E., Manente, R., Dell’Annunziata, F., Folliero, V., Iervolino, D., Casolaro, V., Perrella, A., Santoro, E., Galdiero, M., Capunzo, M., Franci, G. and Boccia, G. 2023. Antimicrobial Resistance in Pseudomonas aeruginosa before and during the COVID-19 Pandemic. Microorganisms 11(8), 1918; doi:10.3390/MICROORGANISMS11081918 Singha, B., Murmu, S., Nair, T., Rawat, R.S., Sharma, A.K. and Soni, V. 2024. Metabolic rewiring of Mycobacterium tuberculosis in response to drug treatment and antibiotics resistance. Metabolites 14(1), 63; doi:10.3390/METABO14010063 Spigaglia, P., Mastrantonio, P. and Barbanti, F. 2024. Antibiotic resistance of Clostridioides difficile. Adv. Exp. Med. Biol. 1435, 169–198; doi:10.1007/978-3-031-42108-2_9/COVER Torres Ortiz, A., Coronel, J., Vidal, J.R., Bonilla, C., Moore, D.A.J., Gilman, R.H., Balloux, F., Kon, O.M., Didelot, X. and Grandjean, L. 2021. Genomic signatures of preresistance in Mycobacterium tuberculosis. Nat. Commun. 12(1), 7312; doi:10.1038/S41467-021-27616-7 Townsend, L., Hughes, G., Kerr, C., Kelly, M., O’Connor, R., Sweeney, E., Doyle, C., O’Riordan, R., Martin-Loeches, I., Bergin, C. and Bannan, C. 2020. Bacterial pneumonia co-infection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC Antimicrob Resist. 2(3), dlaa071; doi: 10.1093/jacamr/dlaa071 Tsai, C.S., Lu, P.L., Lu, M.C., Hsieh, T.C., Chen, W.T., Wang, J.T. and Ko, W.C. 2023. Ribotypes and antimicrobial susceptibility profiles of clinical Clostridioides difficile isolates: a multicenter, laboratory-based surveillance in Taiwan, 2019–2021. J. Microbiol. Immunol. Infect. 57(2), 320–327; doi:10.1016/J.JMII.2023.12.004 Uddin, T.M., Chakraborty, A.J., Khusro, A., Zidan, B.R.M., Mitra, S., Emran, T.B., Dhama, K., Ripon, M.K.H., Gajdács, M., Sahibzada, M.U.K., Hossain, M.J. and Koirala, N. 2021. Antibiotic resistance in microbes: history, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 14(12), 1750–1766; doi:10.1016/J.JIPH.2021.10.020 Unemo, M., Seifert, H.S., Hook, E.W., Hawkes, S., Ndowa, F. and Dillon, J.A.R. 2019. Gonorrhea. Nat. Rev. Dis. Primers 5(1), 79; doi:10.1038/s41572-019-0128-6 Velazquez-Meza, M. East, Galarde-López, M., Carrillo-Quiróz, B. and Alpuche-Aranda, C.M. 2022. Antimicrobial resistance: one Health approach. Vet. World 15(3), 743–749; doi:10.14202/vetworld.2022.743-749 Vendrik, K.E.W., Baktash, A., Goeman, JJ., Harmanus, C., Notermans, D. W., de Greeff, S.C. and Kuijper, E.J. 2022. Comparison of the trends of Clostridioides difficile infections in hospitalized patients during the first and second waves of the COVID-19 pandemic: a retrospective sentinel surveillance study. Lancet Reg. Health 19, 100424; doi:10.1016/J.LANEPE.2022.100424 Woodford, N., Turton, J.F. and Livermore, D.M. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35(5),736–755; doi:10.1111/j.1574-6976.2011.00268.x Xu, Y., Zhang, Y., Zheng, X., Yu, K., Sun, Y., Liao, W., Jia, H., Xu, C., Zhou, T. and Shen, M. 2021. Prevalence and functional characteristics of CrpP-like in Pseudomonas aeruginosa isolates from China. Eur. J. Clin. Microbiol. Infect. Dis. 40(12), 2651–2656; doi:10.1007/s10096-021-04287-2 Zbylicki, B.R., Murphy, C.E., Petsche, J.A., Müh, U., Dobrila, H.A., Ho, T.D., Daum, M.N., Pannullo, A.G., Weiss, D.S. and Ellermeier, C.D. 2024. Identification of Clostridioides difficile mutants with increased daptomycin resistance. J. Bacteriol. 206(3), e0036823; doi:10.1128/JB.00368-23 Zhang, X.X., Zhang, T. and Fang, H.H.P. 2009. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 82(3), 397–414; doi:10.1007/s00253-008-1829-z Zhou, N., Cheng, Z., Zhang, X., Lv, C., Guo, C., Liu, H., Dong, K., Zhang, Y., Liu, C., Chang, Y., Chen, S., Guo, X., Zhou, X. N., Li, M. and Zhu, Y. 2022. Global antimicrobial resistance: a system-wide comprehensive investigation using the Global One Health Index. Infect. Dis. Poverty 11(1), 92; doi:10.1186/S40249-022-01016-5 | ||
| How to Cite this Article |
| Pubmed Style Moosa AS, Jaleel AZA, Ishaq S, Muslim S, Ibraheem S, Naseer MN, Subbaram K, Naher ZU, Faiz R, Huda A, Manandhar PL, Ali S, Tazerji SS, Duarte PM. Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications. J Microbiol Infect Dis. 2025; 15(1): 5-18 . doi:10.5455/JMID.2025.v15.i1.2 Web Style Moosa AS, Jaleel AZA, Ishaq S, Muslim S, Ibraheem S, Naseer MN, Subbaram K, Naher ZU, Faiz R, Huda A, Manandhar PL, Ali S, Tazerji SS, Duarte PM. Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications. https://www.jmidonline.org/?mno=193557 [Access: January 25, 2026]. doi:10.5455/JMID.2025.v15.i1.2 AMA (American Medical Association) Style Moosa AS, Jaleel AZA, Ishaq S, Muslim S, Ibraheem S, Naseer MN, Subbaram K, Naher ZU, Faiz R, Huda A, Manandhar PL, Ali S, Tazerji SS, Duarte PM. Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications. J Microbiol Infect Dis. 2025; 15(1): 5-18 . doi:10.5455/JMID.2025.v15.i1.2 Vancouver/ICMJE Style Moosa AS, Jaleel AZA, Ishaq S, Muslim S, Ibraheem S, Naseer MN, Subbaram K, Naher ZU, Faiz R, Huda A, Manandhar PL, Ali S, Tazerji SS, Duarte PM. Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications. J Microbiol Infect Dis. (2025), [cited January 25, 2026]; 15(1): 5-18 . doi:10.5455/JMID.2025.v15.i1.2 Harvard Style Moosa, A. S., Jaleel, . A. Z. A., Ishaq, . S., Muslim, . S., Ibraheem, . S., Naseer, . M. N., Subbaram, . K., Naher, . Z. U., Faiz, . R., Huda, . A., Manandhar, . P. L., Ali, . S., Tazerji, . S. S. & Duarte, . P. M. (2025) Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications. J Microbiol Infect Dis, 15 (1), 5-18 . doi:10.5455/JMID.2025.v15.i1.2 Turabian Style Moosa, Aminath Shafeenaz, Aishath Zeena Abdul Jaleel, Shifa Ishaq, Saifulla Muslim, Suha Ibraheem, Mariyam Niusha Naseer, Kannan Subbaram, Zeba Un Naher, Razana Faiz, Aminath Huda, Punya Laxmi Manandhar, Sheeza Ali, Sina Salajegheh Tazerji, and Phelipe Magalhães Duarte. 2025. Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications. Journal of Microbiology and Infectious Diseases, 15 (1), 5-18 . doi:10.5455/JMID.2025.v15.i1.2 Chicago Style Moosa, Aminath Shafeenaz, Aishath Zeena Abdul Jaleel, Shifa Ishaq, Saifulla Muslim, Suha Ibraheem, Mariyam Niusha Naseer, Kannan Subbaram, Zeba Un Naher, Razana Faiz, Aminath Huda, Punya Laxmi Manandhar, Sheeza Ali, Sina Salajegheh Tazerji, and Phelipe Magalhães Duarte. "Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications." Journal of Microbiology and Infectious Diseases 15 (2025), 5-18 . doi:10.5455/JMID.2025.v15.i1.2 MLA (The Modern Language Association) Style Moosa, Aminath Shafeenaz, Aishath Zeena Abdul Jaleel, Shifa Ishaq, Saifulla Muslim, Suha Ibraheem, Mariyam Niusha Naseer, Kannan Subbaram, Zeba Un Naher, Razana Faiz, Aminath Huda, Punya Laxmi Manandhar, Sheeza Ali, Sina Salajegheh Tazerji, and Phelipe Magalhães Duarte. "Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications." Journal of Microbiology and Infectious Diseases 15.1 (2025), 5-18 . Print. doi:10.5455/JMID.2025.v15.i1.2 APA (American Psychological Association) Style Moosa, A. S., Jaleel, . A. Z. A., Ishaq, . S., Muslim, . S., Ibraheem, . S., Naseer, . M. N., Subbaram, . K., Naher, . Z. U., Faiz, . R., Huda, . A., Manandhar, . P. L., Ali, . S., Tazerji, . S. S. & Duarte, . P. M. (2025) Narrative review of the silent pandemic: Antimicrobial resistance in bacteria and its implications. Journal of Microbiology and Infectious Diseases, 15 (1), 5-18 . doi:10.5455/JMID.2025.v15.i1.2 |